The growing use of CROs in many areas of product development is well known. Some estimate that up to one-third of drug development activities may soon be contracted out. More recently such outsourcing has extended to other activities, including the optimization of bioproduction materials and processes. This increased use of outsourcing ranges from recombinant clone selection to final product fill and finish.
Both heightened and entirely new process design criteria from a variety of sources are contributing to the increased interest in bioproduction outsourcing. Quality by design (QbD) and operational excellence (OpEx) in general, and such quality initiatives as PAT and ICH Q8–9 in particular, are providing new tools, as well as new goals in bioprocess development. These goals include such exciting prospects as more rapid and comprehensive process monitoring, in-process QC, and real-time release (RTR).
The shift toward platform-type approaches and manufacturing campaigns is driving the requirement that processes be both robust and flexible. In addition, advanced technologies are creating a need for specialized subject-matter experts in some areas of process development.
Advances in cell culture-based production include use of automation in clone selection, disposable formats in standard and flexible seed train design, defined and animal product-free basal production media formulations, high-efficiency materials in fed-batch production, and chemistries that maintain increased product quality through harvest.
Outsourcing’s Value
For many vaccine manufacturers, the emergence of H1N1 has exemplified some of the benefits to be gained from outsourcing. When rapid process development is required, having an active partnership provides an on-call source of experienced shovel-ready capacity. But reduced time-to-market is only one of the many benefits provided by partnering.
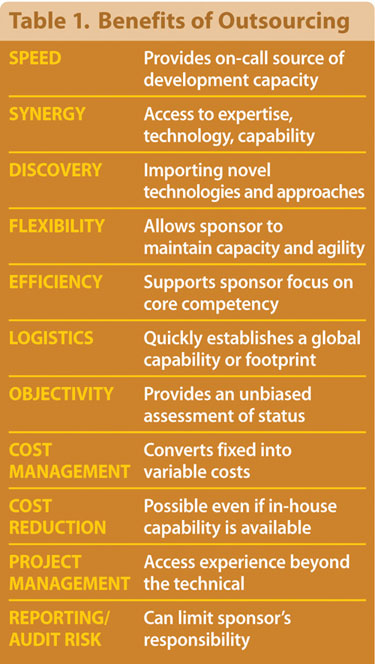
The ability of the sponsor to either maintain a capacity buffer or reduce backlogs as well as the ability to focus on its particular core capabilities are examples of the more indirect value that can be gained by outsourcing. There are also times when a partner’s geography can assist in the transfer or establishment of operations in a new location. Finally, potential financial benefits exist, such as in shifting fixed to variable costs, or if the price of such outside services is lower than in-house costs.
In addition to the immediate project goals, strategic partnering should also take into consideration operational excellence. Quality guidelines that are under development are expected to force the industry to mirror other process-based manufacturing operations that have for years been focusing on minimizing product and process variability.
To become realized, ambitious goals such as continuous quality verification and RTR require access to a number of tools and prerequisite developments.
Once the decision to outsource has been made, a number of factors need to be considered in the choice of an effective partner, including the sponsoring company’s general operating style, its strategic goals, its existing project-relevant capabilities, and specific development or optimization project goals.
The nature and scope of service provider capabilities and characteristics are surprisingly diverse. Depending upon the sponsor’s expertise and goals, firms need to evaluate their potential partners’ attributes, including in-hand process-monitoring technologies, control system expertise, the experience of available personnel, and capacity to manufacture and supply materials defined in the project.
Further Partner Considerations
Outsourced process optimization can require more collaborative activity than other contracted services, as a result efficient operation requires that a number of cultural and geographic factors be at least considered, if not included, in formal search criteria. Experienced contractors know that expertise is just one characteristic of a partner that is capable of providing timely and successful solutions. For example, efficiency of communication, as well as project-specific management and reporting experience can be as important as technical expertise to the completion of a project on time and within budget.
Growing demands within the upstream cell-based bioproduction arena have changed the landscape for material and process development. From the advances in process-monitoring technologies to more capable control algorithms, project planners are struggling to keep up with the increased capabilities now commonly demanded. Bioproduction culture media and feeds are now expected to support not only increased cell densities and volumetric productivity but many other functions.
Thermo Fisher Scientific aggressively addresses these needs by providing total solutions through the integration of cell culture materials, equipment, and services. This begins with the ability to draw upon such immediate capabilities as serum-free media, Single-Use Mixers (S.U.M.), Single-Use Bioreactors (S.U.B.), as well as services provided by the Rapid Response Production and applications development groups. This capability to leverage specific expertise from the broader company at large, such as Thermo Scientific mass spectrometry and protein research products, provides a portfolio of in-house tools to be applied in contracted process optimization.
Case Study 1
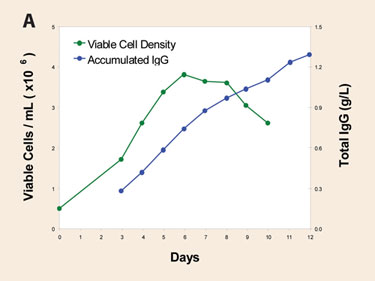
CHO cells are often used for mAb expression. As a result of developments in cloning techniques and media/feeding optimization, production levels from 3–6 g/L are now often expected. Scientists at Thermo Fisher Scientific recently undertook a study to optimize Thermo Scientific HyClone SFM4CHO in order to support the production of over one gram of product per liter with no feeding and minimal process control.
Analyses included extensive characterization of spent media, iterative rounds of multicomponent nutrient supplementation following DoE principals, examination of newly published approaches (such as antiapoptotic chemistries), and prior knowledge from related experience. The results illustrate the accumulation of a mAb in simple batch culture to levels in the area of 1–2 grams/L (Figure 1A).
Case Study 2
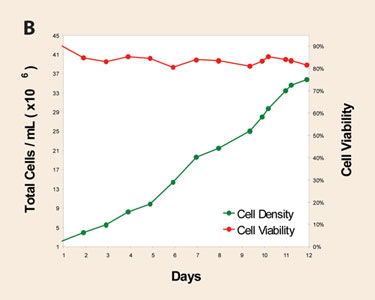
Insect cell culture supporting baculovirus-based expression has been finding more utility in recent years, specifically in viral vaccines. A second study was initiated to develop an animal product-free feeding protocol that would support peak culture densities in excess of 30 million cells/mL in a disposable culture system. The results presented show the application of optimized feed solutions in the fed-batch culture of Sf9 cells using a Thermo Scientific HyClone S.U.B. in Thermo Scientific HyClone SFM4Insect. Peak cell densities in excess of 35 million cells/mL were obtained in well-behaved and scalable disposable systems (Figure 1B).
Many factors are affecting the technologies and expertise required in bioprocess development or optimization, which increases the potential benefits of outsourcing. Both the particular project demands, as well as the existing capabilities and strategic goals of the sponsor company, determine what capabilities should be sought in an outside partner.
In general, a good partner is an international company with a solid business and financial history. More specific criteria include possession of relevant analytics and subject-mater experts, appropriate regulatory certification or license, and demonstrated experience in the operation of interest. But, there are often desirable considerations such as process materials manufacturing and distribution capability, which while ancillary to the project at hand, can contribute to the ultimate economy of the contracted activities.


William G. Whitford ([email protected]) is senior manager of the bioprocessing market for Thermo Scientific cell culture and bioprocessing. Web: www.thermo.com/hyclone.

