December 1, 2011 (Vol. 31, No. 21)
Kathy Liszewski
New Approaches Speed Up Process but Data Analysis Remains Scourge of Many Scientists
With a goal of making assays bigger, better, and faster, multiplexing is gaining momentum as it tackles challenges in the research and diagnostics arenas. The latter market is projected to reach $6 billion by 2013, according to Scienion. Cutting-edge approaches in this rapidly expanding field include new technologies for biomarker development, reducing bias among platforms, improving instrumentation for delivering tiny volumes, and enhancing the rigor of statistical data evaluation.
Some multiplexing assays seek to identify disease-specific biomarkers. This can be a slow and painful process, according to Niro Ramachandran, Ph.D., R&D manager, protein technologies, Life Technologies.
“The only way to speed up biomarker development is to standardize the workflow. It is critical to find out quickly if the lead you are pursuing is valid or not. We have developed a hybrid method for doing this that allows evaluation of more than 50 potential biomarker leads within a three-month period.”
According to Dr. Ramachandran, the company recently developed an antigen-based Luminex assay that complements its ProtoArray® microarray platform. “By coupling these two assays we are enabling everyday scientists to evaluate hundreds to thousands of proteins. Many researchers are familiar with antibody-coated Luminex beads, but a problem with antibody-coated beads is that they can cross react. We prepared beads in the opposite orientation, for example, with the protein targets of antibodies.”
The Luminex xMAP technology itself consists of color-coded beads in 500 distinct sets. Each bead set can be coated with a reagent specific for an assay that subsequently allows capture and detection of analytes within a sample. The ProtoArray is a high-density microarray that contains more than 9,000 unique human proteins spotted onto a 1 inch by 3 inch nitrocellulose-coated glass slide.
“We performed studies that demonstrated how the two technologies in combination (and accessing broad content) could be employed to develop immune response signatures in systemic lupus erythematosus (SLE) and mesothelioma patients.
“For the SLE study, serum samples of 40 SLE patients and 40 matched controls were evaluated on a ProtoArray. This yielded 200 possible markers that were further trimmed to 50 markers. We made proteins to the 50 markers and attached them to Luminex beads. We tested whether these markers were specific for SLE by also testing sera from other diseases such as multiple sclerosis and Crohn disease.
“In the end, we derived 20 markers that performed well and were specific for SLE. This is a great example of how to standardize workflow with high confidence results.”
Although it is possible to utilize several multiplexing platforms to develop and validate biomarkers, a critical factor is how much bias is contained within each platform. “You can lose a lot of time developing biomarkers on several platforms each of which has bias. This compounds the problems and may lead to false positives. We spent a lot of time evaluating this issue and determined that these two platforms nicely complement each other while greatly reducing bias.”
Protein microarrays can also be coated with capture antibodies. LightArray Biotech Research Center uses a 2,470 microarray printing system and can reportedly have up to 16 capture antibodies printed in each well of 96 or 384 plates with strict assay validation to ensure no cross reactivity. The company’s system employs a cool CCD imager and its SignaturePLUS and ProArray analysis software specially designed for chemiluminescent assays. “With this system we are able to provide the quantified protein concentration with a sensitivity averaging 0.1 pg/mL to 1 pg/mL.”
Proximity Ligation Assay
Another approach for high-throughput protein biomarker development is the proximity ligation assay (PLA) that employs antibody pairs bearing strands of DNA forming so-called proximity probe pairs. Simon Fredriksson, Ph.D., CEO of Olink, described a recently developed assay based on biomarker detection via quantitative PCR generated from pairs of target-specific monoclonal antibodies tagged with DNA.
“An enzymatic ligation reaction occurs after the pairs bind their respective analyte in a homogenous solution. This forms a new PCR amplicon composed of reporter sequences of the proximity probes.”
A major limitation of conventional assays is that antibodies may cross react. “Antibodies often do not have perfect specificity. In most multiplex assays, the more antibodies employed the less specificity there is because the probability of cross reactivity increases with greater levels multiplexing. In PLA, each antibody carries a unique DNA sequence that enables the distinct detection of only correct antibody pairs resulting in excellent multiplexing capability.”
Dr. Fredriksson and colleagues validated their assay in pilot studies seeking biomarkers for colorectal cancer using biobanked plasma samples of affected and control populations.
“The platform consisted of four 24-plex panels that profiled 74 biomarkers in the sub-picomole range of sensitivity and utilized only 1 microliter of plasma sample. We were able to identify several potential leads that we will continue to study. Also, we are performing more high-throughput assays utilizing chips and microfluidics. We have increased the assay to a 96-plex assay for our internal use. We plan to go beyond 96 as we continue to refine the assay.”
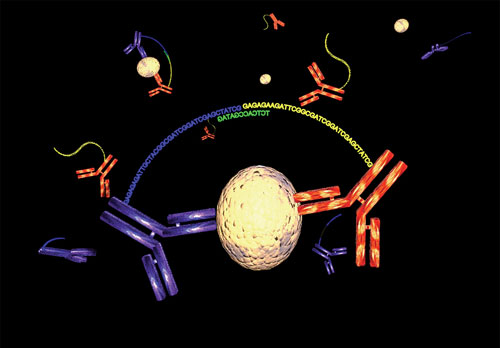
Proximity ligation uses pairs of antibodies equipped with DNA strands that upon simultaneous and proximal binding to the target protein become united, forming a new unique reporter sequence. [Olink]
In Vitro Diagnostics
Although microarrays are widely utilized in research settings, their use in molecular diagnostics has been limited, noted Günter J. Bauer, Ph.D., chief business officer at Scienion.
“As microarrays are entering the diagnostics arena, a number of challenges must be overcome. First, one needs to accurately and reproducibly spot miniscule amounts of functional compounds. The technology also must be affordable. Printing protein arrays on siliconized surfaces is expensive and less amenable to high-throughput analysis. In order to offer a format that is much easier to automate, a feature critical for diagnostic applications, Scienion launched the sciPlexPlate, which it calls a high-quality and flexible microtiter plate.”
The challenge is to spot accurately with the assurance of delivering a functional protein. “Spotting instruments can add 100 analytes quite easily to each well. However, one needs to do this with high precision and reproducibility, especially when applying the very small amounts needed for multiplexing. Our company developed low-volume dispensers that could precisely dispense picoliter to nanoliter amounts of biological materials. We even created a format for using 8-well strips and single breakable well strips allowing the 96-well equipment to be able to prepare this format as well.”
Dr. Bauer indicated that multiplexing the print area is very attractive because laboratories can utilize already available systems they’ve developed. “Many of our clients are developing hits they plan to take to the clinic and employ for diagnostic applications. These applications require miniaturization, automation, and optimization. The challenge is to make them precise and still affordable.”
Rapid ICU Diagnostics
Patients undergoing open-heart surgery or heart/lung transplantation must be temporarily placed on ventilators to assist their breathing. Additionally, other patients, particularly those in intensive care units, may be ventilated. Patients on ventilators run the risk of developing pneumonia.
“Ventilator associated pneumonia (VAP) is the most lethal of nosocomial infections with mortalities running as high as 71%,” reported Jan Weile, Ph.D., project leader, Institute for Laboratory and Transfusion Medicine at the Heart and Diabetes Centre.
“There are at least 375,000 cases of VAP annually in the U.S. and European Union. It can take several days to get results back from a typical bacterial culture. As a result, antibiotics are often begun before the infecting pathogen has been identified. What is needed is a more rapid technology for such diagnostics. In collaboration with Eppendorf Array Technologies, we developed Real-time Array PCR (RAP), a hybrid technology to identify pathogens and antibiotic resistance determinants that combines multiplex PCR amplification and real-time detection on a microarray.
“Our RAP-ID platform is automated and can provide results within two to three hours instead of the two to three days typical of culturing. It consists of a special RAP cartridge with an array with up to 100 targets spotted on the surface of an optical block that is placed into the RAP instrument for automated running and analysis.”
Researchers at the Institute for Laboratory and Transfusion Medicine worked with Eppendorf Array Technology to develop RAP-ID, which quickly identifies pathogens and antibiotic-resistance determinants, the groups report.
Statistical Challenges
Statistical analysis, the scourge of many a scientist, reaches a whole new level when evaluating multitarget clinical laboratory assays. Understanding how to assess performance of these multiplex assays is critical, noted Donna M. Wolk, Ph.D., associate professor, division chair for clinical and molecular microbiology, Arizona Health Sciences Center.
“According to CLIA regulations, verification of new laboratory methods for implementation into a diagnostics laboratory requires a rigorous evaluation of data before tests can be performed on patient samples. When implementing new technology into the laboratory, clinical microbiologists typically use what we call ‘individual analyte’ molecular tests with one or perhaps two genetic microbial target(s). The performance of these tests, compared to a reference standard method, can be done with relative ease. Clinical laboratory directors are generally familiar with the statistics used to characterize and evaluate individual analyte test methods. Now that multi-analyte testing, with detection of 3–25 targets, is a reality, statistical analysis of data presents a new challenge.”
Dr. Wolk says a case in point is multiplex testing for respiratory viruses. “There are several statistical approaches for this multi-analyte testing. One can still analyze the performance of each individual genetic target, which is tedious or one can analyze the entire dataset of results at one time by using statistical analysis like the Chi square test. For either approach, difficulties arise because of the rarity of some virus strains. Data analysis is suspect when laboratories cannot find enough clinical samples to adequately represent all virus categories.”
According to Dr. Wolk, limitations to the breadth, depth, and availability of clinical samples is a challenge. “In some cases limited availability of control material and patient samples leave the laboratorian caught in the space between what is statistically optimal and what is practical in the real world. Inequity exists between large laboratories with abundant samples and smaller laboratories. Microbial biorepositories are limited, and clinical samples are often hard to find. The use of statistics allows us to better define and characterize the limitations in our new method verification and validation.”
Will scientists have to go back to school for years of classical statistical analyses? “Not at all,” Dr. Wolk said. “We need to expand communication between biostatisticians, who have sophisticated training to perform such analyses, and also clinical microbiologists. We can establish guidelines for the statistical practices required for each particular testing application. We need to be able to select and interpret appropriate statistical analyses of clinical datasets for verification and validation of multiplex methods, as well as fine-tune our analysis of qualitative assays, quantitative methods, quality assurance projects, and evidence-based practices.”







