December 1, 2010 (Vol. 30, No. 21)
K. John John Morrow Jr. Ph.D. President Newport Biotech
Non-PCR Point-of-Care Diagnostics and Digital Assays Draw the Attention of Research Scientists
While numerous challenges exist in exploring the biology of disease identification and classification via multiplexing approaches, improvements in high-throughput technology bode well for new clinical offerings. This was one of the main take-home messages at two conferences that looked at multiplexing for research in areas such as cancer biomarkers, HLA typing, and genotyping for human papilloma virus (HPV).
Maurits de Koning, Ph.D., a scientist at DDL Diagnostic Laboratory discussed his company’s efforts at validating a human papilloma virus genotyping assay. The work, which he described at the BEBPA “Biological Assays” conference in Barcelona, is supported by GlaxoSmithKline Biologicals.
HPV is the causative agent of cervical cancer, for which two prophylactic vaccines are now available: Cervarix™ (containing HPV 16 and 18 VLPs) and Gardasil™, encompassing HPV 6, 11, 16, and 18 VLPs. The company’s assay is a PCR-based method, in which cervical biopsy material is obtained from patients, amplified with several primer sets covering 16 HPV types, and then measured using a Luminex-based procedure.
The DDL group constructed a prototype of the assay for proof of concept. Then, during the development phase, the team optimized and designed controls using this platform for evaluation of clinical samples. The next step was the validation of the system according to ICH guidelines.
The elements of validation, from the analytical side, include specificity, sensitivity, accuracy, precision, and robustness. Specificity takes into account the homology of various primers and probes for the many different HPV genomes, given that there may be numerous species that share some of these sequences. Dr. de Koning’s research group evaluated panels of samples and observed that the accuracy was quite high, 98%, when the samples were tested in a comparison with a reference assay.
The precision of the assay, i.e., the degree to which repeat tests of the same material were in agreement, was also quite high, 97%. Another property, that of robustness, or the stability of the assay in the face of minor variations in conditions, was found to be excellent at 95%, with both cervical swabs and formalin-fixed tissues.
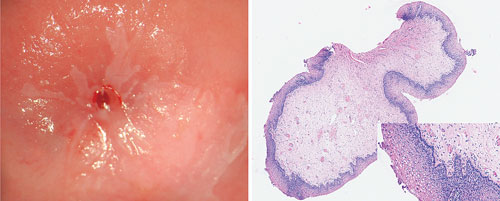
HPV-induced lesions: The left panel shows a macroscopic view of a cervix with the lesion visualized by acetowhite. The right panel shows a hematoxylin and eosin stained section of a formalin-fixed vaginal biopsy. [DDL Diagnostic Laboratory]
Non-PCR Point-of-Care System
Vincent Gau, Ph.D., CEO and president of Genefluidics, spoke about diagnostics for urinary tract infections at the recent “Biodetection Technologies” conference in Arlington, VA. Most current tests are based on cultivating microbes on selective agar and using a tedious antibiotic susceptibility determination.
While bacteriological culture has been accepted as the most sensitive and accurate tool, it is hobbled by a number of shortcomings. A laborious procedure like this sets the stage for the emergence of multidrug-resistant pathogens, as the pathogen proliferates in the intervening period (which can run to several days) between appearance of clinical symptoms and application of therapeutic measures.
Dr. Gau’s company has introduced a molecular-analysis platform that seeks to improve upon traditional diagnostic approaches through development of a handheld point-of-care system. It possesses a universal sample-processing cartridge for conducting multiplexed assays, either nucleic acid or immunoassay-based, and is field deployable.
The current unit was introduced in 2010 and is extremely compact. It uses exchangeable cartridges which are constructed with channels through which the reagents are pumped. Detection of the signal is through the use of flow impedance. When antibodies, small molecules, or nucleic acids bind to the surface they affect the current flow, which is recorded as a change in impedence in the chip that carries the sample.
According to Dr. Gau, the device produces highly accurate data with tight error limits associated with the measurements. A variety of biological samples have been evaluated including blood, serum, urine, saliva, cerebrospinal fluid, sputum, milk, and meat juices. A wide range of markers have been controlled using the system—IL-8, tropinin, numerous bacterial species, and multiple drug-resistance markers.
“I believe that these products will enable clinical diagnosis to move to a new level of performance, with attendant cost savings and health benefits for our customers,” said Dr. Gau.
Assays Go Digital
Applied Biocode offers a barcoded, magnetic-bead, high-throughput approach based on digital technology, according to company president, Winston Ho, Ph.D., who was one of the speakers at the “Biodetection Technologies” meeting.
“The motivation for development of our platform was the need to process a large number of tests—up to 1,024—in a single microwell.”
The barcodes the company uses are similar to those found in other commercial operations. The number of permutations of arrangements of the barcode is 2N, so that by increasing the number of wide and narrow bars in a module from 1 to 10, the number of unique identifiers can be easily extended.
The company’s technology employs a permanent digital barcode, which is bonded to the beads using photolithography for greater batch consistency. It is open ended, scalable, and the detection of the signal is accomplished with an LED and a lamp, rather than through the use of lasers and flow cytometers, thus providing greater robustness, according to Dr. Ho.
Because of the elevated accuracy of the barcode classification, a large number of different beads can be loaded in a single microwell. Positive reactions are detected using fluorescence with the dye, such as phycoerythrin, which fluoresces at 575 nm.
By combining digital barcodes with molecular diagnostics, the company has built a polymer bead with the magnetic barcodes encapsulated inside and permanently encoded. This accuracy allows up to 1,024 tests to be combined in a single tube.
Applied Biocode is in collaboration with a number of partners to develop various DNA, RNA, and protein assays. These include IL-2, with a sensitivity down to the pictogram range, MRSA, and a ten-plex panel for cytokines.
GE Global Research (ge.geglobalresearch.com) has developed a lead technology platform for measuring multiple biomarkers in tumor tissues, explained John Burczak, Ph.D., chief scientist for molecular imaging at the company. His team is evaluating methods for the detection of cancerous pathology in tissue samples.
The conventional approach, when suspected malignant tissues are scored, is by a somewhat subjective judgment of the pathologist, who ranks the tissues on a numerical scale according to the degree of malignancy. The decision is frequently controverted by alternative expert opinion, and in some cases by the original evaluator on re-examination.

BioCode-1000 Analyzer is a barcoded magnetic bead optical imaging system for a 96-well microplate format. After undergoing reaction, the beads settle to the bottom of each well, allowing for barcode identification and fluorescent signal detection. Multiple analyte tests can be performed in each well. [Applied Biocode]
Multiple Biomarker Analysis
A more objective approach is the use of antibodies directed against cancer-associated proteins, reacted with tissue homogenates, but the strategy only provides an average over the entire sample, rather than revealing tiny microfoci of cancerous tissue within. Given that pathology samples are extremely heterogeneous, containing necrotic, normal, and transformed cells, this method may miss important features of the test material.
The approach adopted by Dr. Burczak’s team is based on the use of tumor biomarkers detected by immunocytochemistry. Four different antibodies are coupled to four different fluorescent dyes. The tumor slices are stained and the image is quantified using digital microscopy. The dyes are quenched with a mild agent that does not damage the proteins, and the entire process is repeated. Using this serialized approach it is possible to follow up to 100 biomarkers on the same slice of tissue, according to Dr. Burczak.
“To avoid loss of information, we test the most fragile proteins first and then work up to the more robust markers that have no chance of sustaining damage by multiple quenching steps. Essentially, we can do what cell sorting does but on a solid tissue.”
The approach also allows evaluation of the status of individual cells or even regions within the cells. By varying the level of magnification from 5x to 20x through 40x it is possible to visualize the abundance of proteins within the cytoplasm or other regions within the cell. “We are probing deeply into subcellular quantitative analysis using tissue slices,” reported Dr. Burczak.
As an example of the platform’s applications, he mentioned the Her-2 neu protein, the estrogen receptor critical for binding of the antitumor agent tamoxifen. Tumors that do not have estrogen receptors do not respond to such therapies, and prior to the development of these detection systems it would not have been possible to decide on the appropriate course of treatment for breast cancer patients.
K. John Morrow Jr., Ph.D. ([email protected]), is president of Newport Biotech and a contributing editor for GEN.







