May 1, 2017 (Vol. 37, No. 9)
MaryAnn Labant
The Most Interesting Thing about CRISPR/Cas9 Is What It Can Accomplish in the Hands of Gifted Researchers
The tooth-to-tail ratio, or the balance of frontline troops to support echelons, can determine success or failure in any complicated operation. Originally a military notion, the tooth-to-tail ratio also applies to commercial and scientific endeavors, such as the routinization of genome editing. Ideally, genome-editing technologies such as the CRISPR/Cas9 system should become so routine that they fade into the background.
Although CRISPR/Cas9 is a fascinating technology, the most interesting thing about this cutting and pasting machine, this RNA-guided endonuclease, is what it can accomplish in the hands of gifted researchers. Eventually, CRISPR/Cas9 will become just another tool, and everyone will go about their lives without having to hear about how “hot” it is. Before that happens, however, the other end of the CRISPR/Cas9 spear will need more attention and development. That is, CRISPR/Cas9 will need better supply, upkeep, and logistics.
Both parts of the CRISPR/Cas9 spear—the pointy research bit and the large support structure—were discussed at the CRISPR Precision Genome Editing Conference. This event, which recently took place in Boston, highlighted some exciting applications. What’s more, it included presentations that brought to mind the famous dictum, “Amateurs talk about tactics, but professionals talk about logistics.”
Cross-Species Transplantation
One application benefiting from CRISPR/Cas9 technology is xenotransplantation, or cross-species transplantation. It offers the prospect of an unlimited supply of organs and cells, and it could resolve the critical shortage of human tissues.
For ethical and compatibility reasons, xenotransplantation shifted away from nonhuman primates as a potential source of donor tissues. Instead, the discipline began to focus on porcine organs. Nonetheless, in 1997, pig-to-human transplants were banned worldwide due to concerns about the transmission to humans of porcine endogenous retroviruses (PERVs), which are integrated into the genome of all pigs.
According to George Church, Ph.D., professor of genetics, Harvard Medical School, work was undertaken in his laboratory on PK15 porcine kidney epithelial cells to determine if PERVs could be eradicated. It was crucial to avoid disrupting the envelope gene and the terminal regulatory elements, as both of these could be important during normal pig fetal growth. In addition, a highly conserved target in the viral polymerase gene was desired for the guide RNA (gRNA) to bind.
First, the copy number of PK15 PERV was determined to be 62. Then, when CRISPR/Cas9 was used along with two gRNAs, one which did the bulk of the work, all 62 copies of the PERV pol gene were disrupted, demonstrating the possibility that PERVs could be inactivated for potential clinical pig-to-human xenotransplantation. The repeats were well separated, and not clustered, which could have meant higher toxicity.
After two weeks of cell culture, about 8% of clones were 100% altered, and no rearrangements were found. Although a few off-target effects and point mutations were expected, they were deemed unlikely to have an impact on pig fetal development. As with conventional breeding, PERV-free clones were empirically selected as they were the healthiest.
In addition to disrupting dozens of endogenous viral elements, Dr. Church’s group altered dozens of genes involved in immune and blood-clotting functions to increase human compatibility. Some of the changes were so extensive that more powerful DNA recombination tools, and not CRISPR, were utilized.
This work may benefit eGenesis, a Cambridge biotech focused on leveraging CRISPR technology to deliver safe and effective human transplantable cells, tissues, and organs. eGenesis was cofounded by Dr. Church and Luhan Yang, Ph.D., in early 2015 and is based on their research.
Ex Vivo Indications
Another emerging company working on CRISPR clinical applications is CRISPR Therapeutics. This company’s initial emphasis is on ex vivo indications. Ex vivo indications have the benefit of a facile delivery approach, such as electroporation, and the ability to characterize the edits before administering treated cells to the patient. Plus, measuring biomarkers to understand phenotypic effects in a relatively short timeframe after therapy administration is straightforward.
The company’s lead indication, a compound to treat inherited single-gene hemoglobinopathies (such as sickle cell and beta thalassemia), relies on gene disruption to upregulate fetal hemoglobin. This approach could be curative. A large number of studies show that patients who have sickle-cell or beta-thalassemia traits are asymptomatic when they have upregulated fetal hemoglobin.
“We can achieve gene disruption today using CRISPR/Cas9 with relatively high efficiency, more than 80–90%,” asserted Sam Kulkarni, Ph.D., chief business officer, CRISPR Therapeutics. “Gene correction approaches are being improved continuously, but the efficiency of correction is still in the 50–60% range for hematopoietic stem cells.”
The key challenges to overcome include delivery, pharmacology, and manufacturing. CRISPR/Cas9 is a multicomponent system and needs to be delivered to the target organs or tissues for in vivo applications. Previous work on small interfering RNA (siRNA) and other therapeutic modalities may prove beneficial.
Careful analysis is required to characterize the type of edits and the fraction of cells edited. This pharmacology hurdle is easier to clear ex vivo than in vivo. Finally, manufacturing involves multiple components and also complex cell manufacturing. Collective efforts of private and academic laboratories are rapidly surmounting these issues.
Recent advances that result in high levels of homology-directed repair are facilitating efforts to expand the addressable indications with CRISPR. Some approaches attempt to impact the level of cycling of the cells; others utilize modifications of the donor template and guides; and yet others are working on optimization of the process of CRISPR/Cas9 directed repair.
Arrayed CRISPR Libraries
Tools play an important role in making the prospect of high-throughput knockout screening a reality. Such tools have been pioneered by MilliporeSigma, which has launched various CRISPR products. The first such product consisted of simple constructs/plasmids that could accommodate targeting elements and yield custom clones. Soon after developing this product, MilliporeSigma realized that a large collection of clones would be of great utility.
A previous collaboration between MilliporeSigma and the Broad Institute had resulted in a short hairpin RNA (shRNA) library, and the goal was to duplicate that model to develop a product that would work for the majority of researchers.
Another collaboration, this one between MilliporeSigma and the Wellcome Trust Sanger Institute, had a similar vision, and after two years of work, it generated its first complete arrayed whole-genome CRISPR screening libraries. The off-the-shelf libraries offer substantial cost savings and facilitate standardization of CRISPR screening. Although pooled libraries have been available for a while, the arrayed libraries provide one clone for one gene in one well, reducing ambiguity about the target at screen completion.
The human and mouse libraries were designed to knock out virtually every protein coding gene in their respective genomes, and each library contains two unique and highly specific gRNAs for every gene target. The second clone is used to verify that the first is not an artifact or a false positive.
The human library contains approximately 34,000 clones targeting 17,000 genes, and the mouse library contains approximately 40,000 clones targeting 20,000 genes. Bacterial glycerol stock, plasmid DNA, and lentiviral formats are available.
“The beauty of the Sanger designs is that they will hit that gene and that gene only,” commented Shawn Shafer, Ph.D., director, advanced genomics, MilliporeSigma. “Anything that did not meet our stringent design strategy did not make it into the library.
“Some genes are tiny or highly repetitive, and so these genes were not suitable targets. Now you can screen for one gene, a couple, or the whole genome.”
The arrayed library will continue to evolve. Dialing a gene up or down is looking plausible, and the libraries may be developed for use in gene activation and repression.
MilliporeSigma participated in the development of the Sanger Arrayed Whole Genome Lentiviral CRISPR Libraries. According to the company, these are the first commercially available off-the-shelf arrayed lentiviral CRISPR gene knockout libraries for screening human and mouse genomes. The genome-wide loss-of-function screens won an R&D 100 Award in 2016.
Pooled CRISPR Libraries
Highly complex libraries of oligonucleotides, including pooled CRISPR libraries, require the synthesis of hundreds to hundreds of thousands of unique oligos in a single library. When produced using a traditional, column-based approach, each individual oligo needs to be pooled following synthesis, opening the door to errors.
Column-based synthesis is not the best approach for pooled CRISPR libraries. With recent developments, Agilent’s synthesis fidelity has increased significantly with the ability to synthesize libraries in which it is difficult to differentiate errors from synthesis versus sequencing errors.
The throughput of the platform combined with tight spatial control allows active tuning of biases within every library. Both the chemistry and the process to print DNA have been refined, and biases are measured using methods such as DNA sequencing.
“The quality difference is apparent in many of our oligo-based products but most recently in our catalog and custom SureGuide pooled CRISPR libraries,” stated Ben Borgo, Ph.D., global senior product manager, diagnostics and genomics division, Agilent Technologies. “The ability to generate libraries with uniform, even representation is directly tied to the spatial control and accuracy of the DNA printers.”
A process derived from inkjet printing is used to deposit nucleotides to a growing chain on a solid support. Miniature jets contain the different dNTPs (nucleoside triphosphates containing deoxyribose), other monomers, and chemicals. These nozzles place droplets of reagent into specific sites on the solid support.
A generalized workflow allows researchers to design their own library or utilize Agilent’s designs, synthesize the library quickly, inexpensively generate a plasmid library that maintains or improves the quality of the synthesized library, and then move downstream to begin addressing specific questions. In addition, a library-cloning technique that eliminates the need for agar plates has been developed.
As new Cas9 variants are being described, each application requires a new library. Libraries have already been developed for some of these techniques, and will continue to evolve and change as needed.
CRISPR Mouse Models
In the late 1980s, pronuclear injection was first performed to make transgenic mice. The ability to modify an endogenous gene in the mouse genome came later. It was achieved by homologous recombination in mouse embryonic stem (mES) cells that were injected into blastocyst embryos to create chimeric mice. These chimeric mice could be bred to produce mice derived solely from the mES cells.
This inefficient process requires selection, often leaves behind undesired genetic material, and relies on skilled mES cell culturing so that they retain pluripotency and can populate the germline.
Initially, mES cells were isolated from the 129 mouse strains. More recently, they were isolated from other stains. Creating genetically modified mice on other background strains requires a long backcross breeding process.
“With CRISPR and nuclease-assisted homologous recombination we can go directly into the embryo and create the desired mutation directly on many mouse inbred strains,” discussed David Grass, Ph.D., senior director, genetic engineering, The Jackson Laboratory. “In addition to performing projects on C57BL/6J and BALB/cJ, we have even been able to work in the immune-deficient NSG mice.
“NSG mice carry two mutations including severe combined immunodeficiency (SCID) known to be involved in DNA repair. So we were not sure how efficient the CRISPR would be. But it has worked really well, allowing us to work on the ‘next generation’ of humanized mice.”
It used to take 1–1.5 years to produce a germline mouse; backcrossing took another 1–1.5 years. With CRISPR, it takes 6–8 months. From a cost perspective, for simple projects, such as small indels, CRISPR is 81% less costly; for the more complicated homologous recombinations, it is 40% less costly.
Fortunately, if off-target events occur in mice, they can be segregated from the desired allele when the mice are bred, unless the off-target is closely linked in the genome to the desired mutation.
The Jackson Laboratory is currently working on understanding how to titrate gRNA/Cas9 activity and to make the process more efficient. For example, efficiencies could be realized though techniques such as batch electroporation, which can introduce reagents into embryos.
Knowing When CRISPR Is the Right Approach to Generate Your Model
Demand for obtaining genetically engineered animal models on faster timelines has fueled the development of gene-editing technologies like CRISPR. As interest skyrockets, it is critical to evaluate how and where to employ CRISPR in the development of animal models for in vivo studies.
The simplicity and efficiency of CRISPR, and the promise of shorter lead times, have led investigators to push the limits of this technology. In certain applications, CRISPR does enable generation of a model much faster compared to using embryonic stem (ES) cell-based methods. Yet, CRISPR isn’t necessarily the best approach for every project.
Taconic Bioscience’s experience indicates that CRISPR works extremely well for generating simple allelic configurations such as constitutive knockouts and knock in of point mutations, according to Adriano Flora, Ph.D., associate director, scientific program management, at the company.
“However, it is not as well suited to introducing more complex modifications relying on homologous recombination over larger regions,” he says. “While Taconic and others have done the latter successfully, the complexity of the effort can create longer and unpredictable timelines and higher costs, potentially negating the reasons for selecting CRISPR in the first place.”
Dictating Genetic Modifications
The balance of CRISPR’s advantages with its limitations dictates the kind of genetic modifications that can be introduced and yields a subset of research projects for which the technology is most appropriate for developing a suitable animal model.
An interesting application is model refitting: the introduction of additional genetic modifications to well-established existing models where multiple modified alleles are already present, or the modification of transgenic alleles in already established mouse lines.
“The latter application is ideal for testing the efficacy of a therapy on models that carry different variations of a humanized gene,” says Dr. Flora. “For example, Taconic has used CRISPR to develop mouse models with varying metabolizing levels of a human liver gene involved in drug metabolism, mimicking natural variants present in the human population.”
In the final analysis, successful use of CRISPR to generate animal models demands proper evaluation of the specific research objective and project requirements, followed by selection of the most appropriate tool from the model generation toolbox.
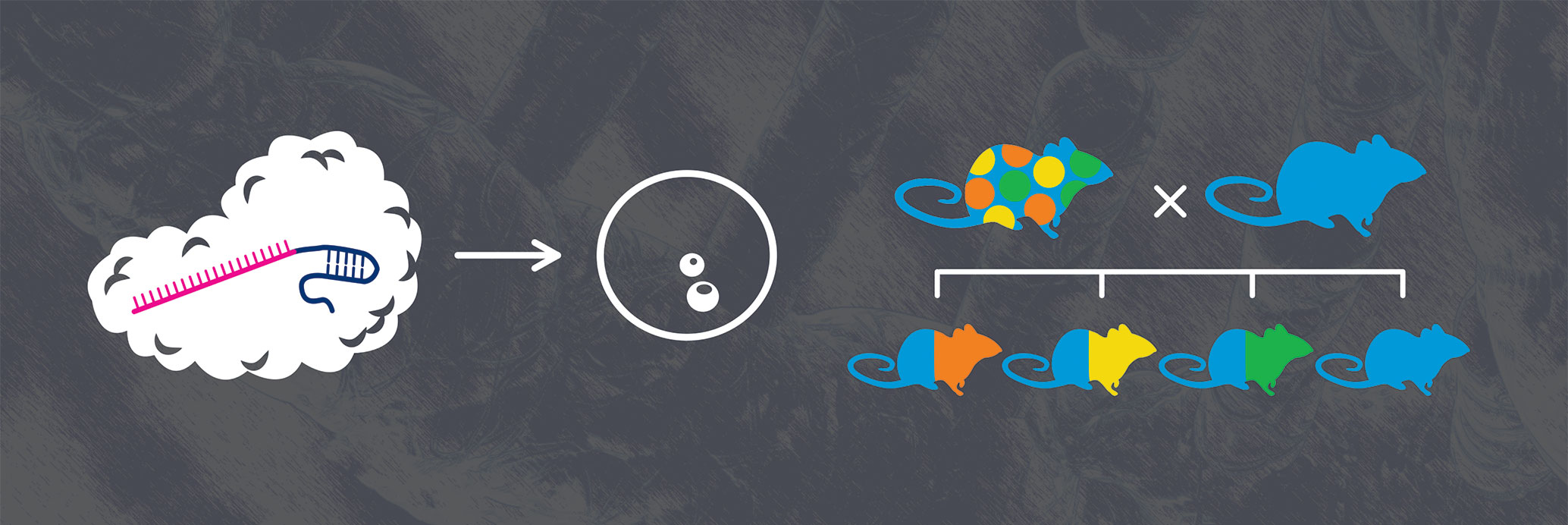
To perform genetic modification on a mouse strain using CRISPR, single guide RNA(s), Cas9 mRNA or protein, and a targeting vector (depending on the nature of the desired mutation) are microinjected into pronuclear stage embryos. After genotyping/sequencing analysis, the founder mice are backcrossed to mice of the appropriate background strain to generate mice that carry the mutations. Founder mice are often mosaic (as shown by the multicolored mouse). As a result of this mosaicism, it is not always easy to achieve germline transmission of a particular mutant allele. However, once the allele has been transmitted through the germline, the allele will be transmitted as expected by Mendelian genetics. [The Jackson Laboratory]