March 15, 2013 (Vol. 33, No. 6)
Vicki Glaser Writer GEN
No aspect of biopharma better epitomizes the goals and promise of delivering personalized medicine to treat and perhaps even cure intractable and disabling diseases than the emerging fields of cell and gene therapy.
Adding to the excitement surrounding these technology-driven treatment approaches are their recent advance to the marketplace and increasing examples of progress in meeting clinical-scale production and regulatory challenges and achieving commercial success.
The progress and opportunities, obstacles and solutions, and optimistic projections and cautionary portents were all part of the science, technology, economic analysis, and insights presented, debated, and discussed at the recent “Phacilitate Cell & Gene Therapy Forum”.
Mahendra Rao, Ph.D., director of the NIH Center for Regenerative Medicine and head of the Laboratory of Stem Cell Biology at the NIH, led off the meeting and highlighted a key distinction from previous years: the growing number of commercial products now available for regenerative medicine.
Success begets new challenges, however, and Dr. Rao identified some of the issues this new reality is creating for young biotechnology companies. These may include how they ship their product, how to make a profit, how to deal with reimbursement and insurance issues, and how to manage competition.
The unique characteristics of cell therapies present new hurdles for this fledgling industry sector: individualized products prepared in small and even single doses; entirely or largely manual processes that need to be automated; and the challenges associated with reducing the cost of goods and leveraging economies of scale.
Other critical factors related to the actual products include storage, transport, time- and temperature-sensitivity, and time-out-of temperature specifications; chain of custody, overall quality control issues; and logistics considerations across the supply chain.
The main take-home message was to start thinking about factors related to commercialization of a cell therapy product early in its development, and at every step along the way—including, for example, the potential impact of a change in a process, protocol, raw material, or analytical method.
Also emphasized by several presenters was the inevitable move toward automation, in the short-term at least aimed primarily at allogeneic cell therapies. Automated processing of cells and cell-based products will be essential for achieving optimal quality control and standardization, designing closed, disposable process streams, eliminating the risks associated with manual intervention and open systems, and reducing costs.
Scientists at UC-San Diego have used mice’s own red blood cells to create nanoparticles for delivering anticancer therapeutics to tumors in their bodies. [Fusebulb/Fotolia]
Moving to the Market
A roundtable discussion among a group of industry executives who have navigated the process of cell therapy commercialization focused on the question, “what does a commercially successful cell or gene therapy look like?”
One example was CartiStem®, developed by Medipost, and approved in 2011 in Korea for cartilage regeneration in the treatment of traumatic and degenerative osteoarthritis. The product has been used in about 250 patients but is not yet reimbursable. CartiStem is in Phase II trials in the U.S.
John Maslowski, vp scientific affairs at Fibrocell Science, which markets the aesthetic autologous human dermal fibroblast product azficel-T (LAVIV®) to improve the appearance of wrinkles, spoke about the labor-intensive nature of current autologous cell manufacturing processes.
Geoff MacKay, president and CEO of Organogenesis, described the company’s semi-automatic manufacturing process in place to produce its recently approved Gentuit™ product, composed of allogeneic human keratinocytes and fibroblasts, for oral tissue regeneration around teeth and implants.
For TiGenix, key hurdles in bringing to market its ChondroCelect® autologous cartilage product for the repair of single symptomatic defects have included lack of regulatory harmonization, and the need to reduce the cost and increase the effectiveness of therapy. Access drives uptake, the company has found, and its commercialization strategy has included offering regulatory authorities cost controls and volume caps, identifying and targeting patient subgroups likely to have the best outcomes, and pursuing a stepwise, managed market introduction. TiGenix presents the product to physicians and regulators as a value proposition, with evidence-based results and support services.
Driving Market Uptake
Reed Tuckson, M.D., evp and chief of medical affairs, UnitedHealth Group, one of the largest healthcare insurers in the U.S., told the attendees, “We believe you need to succeed and we believe deeply in innovation.” It is important to facilitate access to these new products and technologies, he added, but at the same time, he expressed real concern about the dire implications of escalating healthcare costs in the U.S., for which consumers will bear the burden going forward.
There is too much waste in the system now, noted Dr. Tuckson, and too many aspects of healthcare are poorly managed. The prospect of adding increasingly complex and costly therapies into the mix, such as cell therapies, is extremely worrisome from a payer’s perspective. It would not be acceptable to waste or misuse these potentially life-saving innovations.
Dr. Tuckson described three emerging strategies essential for the future of the healthcare industry: value-based designs that emphasize individual accountability; value-based reimbursement design that will replace the fee-for-service paradigm; and value-based technology assessment, in light of optimizing outcomes and looking at the total cost of care—for example, a treatment that can provide a lifelong cure vs. a lifetime of medication, and options that allow for oral vs. parenteral therapy or outpatient vs. inpatient treatment.
He encouraged companies developing cell therapies to begin talking to insurers early. “We need to know how it will work and how it will be used in the real world,” he said, urging companies to provide data, identify an optimal patient population, and develop unit cost projections.
Automation Is Critical
From the perspective of efficiency and economy, the consensus among presenters was that manufacturing of cell-based therapies will have to move toward more automated processes. Automation and closed process streams are becoming more commonplace in the allogeneic cell therapy setting, and autologous product development strategies will have to follow suit. Even with autologous cell therapy, companies need to think in terms of volume, scale, and platform technologies, urged the speakers. Scale, however, need not necessarily imply scale-up, as in the traditional pharmaceutical manufacturing model, but instead scale-out, producing multiple individualized products in parallel.
Overall, the presenters were optimistic that cost would not be an obstacle to successful commercialization of cell therapies in the future, and attractive cost/benefit ratios will be achievable. They offered insights and suggestions for minimizing cost of goods, maximizing efficiency of operations, developing best practices, and optimizing manufacturing and business models.
Knut Niss, Ph.D., senior R&D manager, Stem Cell Initiative, EMD Millipore, distinguished between the needs in producing an autologous cell therapy—low cell numbers, a fast process, and one manufacturing run/patient—and an allogeneic product, in which the aim is to generate as many doses as possible in a single batch, with the goal of moving toward bioreactors for large-scale manufacturing.
Kim Warren, head of development services for cell therapy at Lonza, commented on one of the challenges in moving the production of adherent cells to bioreactors and the use of microcarriers to increase cell yields. Ideally it would be better to move away from microcarriers and go to cell clusters, but that is “easier said than done,” Warren said. Another challenge is how to isolate the cells from the large volumes used in bioreactors. “I don’t think we have the downstream technology yet,” added Warren.
An important target for reducing the cost of goods in allogeneic cell therapy is the media in which the cells are grown, and decreasing or eliminating serum and other animal-based components. Warren noted, however, that these would have to be replaced with recombinant growth factors and supplements, and the cost of those remains relatively high due to limited demand. We are caught “between the problem of supply and demand,” Warren said. Another concern when bovine serum and animal proteins are removed is whether the cells change in any fundamental way.
Frederick Miesowicz, COO and vp of manufacturing at Argos Therapeutics, presented a case study summarizing the do’s and don’ts of an automated approach for scale-up of personalized immunotherapies. Argos is automating its Arcelis personalized immunotherapeutic platform technology. The method involves capturing all of the unique antigens from a patient’s tumor and developing an immunotherapeutic agent that targets the patient’s mutated tumor antigens without provoking an autoimmune reaction, thereby sparing the healthy tissue. The company has a product in a Phase III trial in renal cancer.
“Start thinking about automation even before you know the clinical efficacy of your product if you expect it to be ready by the time you get to Phase III,” said Miesowicz. With automation, he is confident that cell therapies will be able to achieve costs that are in the range of existing cancer drugs.
Argos uses mRNA technology to target the dendritic cell-based vaccine. The vaccine is stored in liquid nitrogen and can be maintained for five years. Vaccine is prepared individually for each patient, initially through a fully manual process that involves extracting DNA from the renal tumor, preparing the mRNA through reverse transcription and PCR, and preparing the dendritic cells from a leukophoresis sample from the patient.
On a large scale, however, vaccine preparation will not be feasible as a manual process and will require automation. “This is a scale-out, not a scale-up scenario,” said Miesowicz. Automation with a closed disposable process stream will be essential for consistency, to decrease the size of the facility and the number of personnel required, and to eliminate the possibility of cross-contamination and provide segregation between processes and products.
The automated system encompasses three units: one for cell preparation; one for producing amplified tumor RNA, with a robotic arm and sterile connector, and only the equipment interacts with the patient material; and one for performing electroporation and finish/fill steps.
ISTO Technologies, an orthobiologics company, has two cell-based allogeneic products in development. Its De Novo® ET product for cartilage regeneration is in a Phase III trial for knee injuries in sports medicine. ISTO is working with juvenile chondrocytes, which are up to 100-fold more efficient than adult cells in producing new cartilage tissue, according to Mitchell Seyedin, Ph.D., president and CEO of ISTO. The company’s cell expansion platform involves creation of a cell bank by growing chrondrocytes in a bioreactor of its own design using a scaffold-free method.
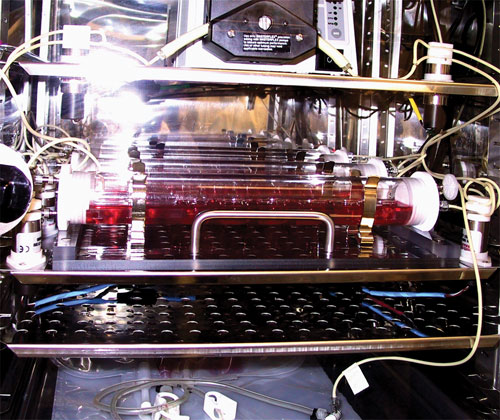
ISTO’s cell-based orthobiologic platform uses juvenile cartilage cells to create products designed to repair and restore function to damaged cartilage tissue.
Illustrating how automation can streamline cell procetssing for clinical adoptive immunotherapy applications, Kai Pinkernell, M.D., global head of clinical business at Miltenyi Biotec presented a semi-automated scenario involving a five-step process that begins with antigen stimulation of cells and culminates with interferon-gamma enrichment reagent labeling and magnetic selection and elution. For a GMP lab, he estimated that this semi-automated approach would require two to three days of work, with a throughput of two runs/week per full-time employee (FTE). Using a fully automated system such as Miltenyi’s CliniMACS® Prodigy single-use disposable platform, cell processing in a GMP environment would entail one day of work, with a throughput of five runs/week/FTE and reduce cleanroom requirements and hands-on time.

Miltenyi Biotech’s CliniMACS® Prodigy reportedly offers advanced integrated solutions to streamline cell-processing workflows: from cell fractionation through cell culture to formulation of the final product.
Managing Risk
In a workshop led by Thermo Fisher Scientific, Joseph Granchelli, Ph.D., manager of applications and technical support, introduced the company’s new department, “Customer Applications and Compliance Support,” which encompasses the company’s materials and regulatory compliance groups.
Dr. Granchelli noted that as cell therapy production moves into automated, single-use disposable process streams, one over-arching concern relates to the potential for polymeric materials used to make the single-use bags, connectors, and tubing, for example, to be resorptive, reactive, or additive and to adulterate the final product. Further confounding this issue at present is a lack of clarity and standardization on how and when to test for these effects, what to look for, and what the results mean. Regulatory bodies have not established specifications for how much testing may be required and how to go about doing it.
The presentation focused on the potential for single-use materials to be additive and, specifically, on two problems. The first is extractables—compounds forced to migrate from a molded product under highly exaggerated conditions, such as exposure to “aggressive” solvents, extreme temperatures, sterilizing irradition, high pressure, the presence of stabilizers, colorants, and plasticizers, for example, and factors related to packaging that can lead to off-gassing. The second concern is leachables—materials that migrate into the final product or an intermediate at typical or somewhat exaggerated conditions.
Dr. Granchelli presented a risk-based approach that involves assigning a risk for each component to the final product and, where there may be risk, determining if extractable/leachable data is already available and otherwise performing the appropriate assays. He described how vendors can help with this approach if they have already tested their single-use products under a range of conditions and can make the data available to customers.
10 Most Significant Events in Cell Therapy in ’12
Ed Field, vice chairman of the Alliance for Regenerative Medicine (ARM) and COO of Cytomedix, counted down the top 10 most significant events of 2012 in the cell therapy field:
10. U.S. District Court (for the District of Columbia) decision in July in favor of the FDA, granting its motion for summary judgment against Regenerative Sciences and issuing a permanent injunction against the use of the company’s Regenexx Procedure, which involves removal, culture, and reinjection of a patient’s stem cells to treat joint, muscle, tendon, or bone pain; Regenexx argued the treatment is a procedure, while FDA contended it is a drug.
9. Cytori was awarded an up to $106 million contract by Biomedical Advanced Research and Development Authority (BARDA) in September to develop cell therapies for the treatment of thermal burns combined with radiation injury; about $80 million of total is for clinical work.
8. October launch of StemBANCC, a five-year research program to develop human induced pluripotent stem cells as a platform for drug discovery; includes 10 major pharmaceutical companies and academic institutions from 11 countries.
7. Publication of promising Phase I and interim results for central nervous system and eye disorders, including results from Advanced Cell Technology from clinical studies of its human embryonic stem cell (hESC)-based retinal pigment epithelial therapy to treat macular degeneration; interim results were reported for the pilot investigation of stem cells in stroke trial using genetically engineered neural stem cells to treat ischemic stroke earlier this year; and Phase I goals were met in a study of lumbar intraspinal injections of fetal-derived neural stems cells in patients with amyotrophic lateral sclerosis (ALS), for example.
6. Smith and Nephew announces acquisition of bioactive woundcare company Healthpoint Biotherapeutics for $782 million in November.
5. Organogenesis’ Gintuit cell therapy product for oral tissue regeneration received FDA approval in March; first cell-based product from allogeneic human cells and bovine collagen; for topical application to surgical site to treat mucogingival conditions.
4. Cell therapy and cell-based regenerative medicine raised more than $1 billion in 2012, based on all forms of funding.
3. Approval of Osiris Therapeutics’ Prochymal stem cell therapy for the treatment of acute graft-vs-host disease in children in Canada in May and in New Zealand in June.
2. John Gurdon, University of Cambridge, U.K., and Shinya Yamanka, Kyoto University, Japan, are awarded the Nobel Prize in Physiology or Medicine.
1. University of Pennsylvania and Novartis sign a licensing deal valued at $20 million to develop an autologous T cell-based immunotherapeutic for cancer based on chimeric antigen receptor technologies.