November 1, 2015 (Vol. 35, No. 19)
Bill Gigante Product Manager/Mechanical Engineer Stratec
Tanya Knaide Product Manager Artel
John Thomas Bradshaw Ph.D. Senior Development Scientist Artel
Common Plate-Handling Methods May Result in Unexpected Volume Changes
Thanks to equipment advances made to meet the demands of high-throughput screening, the use of automation in modern drug discovery and research labs has become commonplace. However, as sample density increases in screening novel drug candidates, humans become more removed from the screening process and proper sample care can occasionally be overlooked.
One of the primary sources of random error can be attributed to environmental factors such as humidity and temperature within the laboratory. Sample plates are commonly unsealed or de-lidded and stacked within a carousel on a robotic platform or loaded onto the deck of a high-speed liquid-handling robot. In many cases, these plates are left exposed to the laboratory environment for hours at a time, and when this occurs, the last plate processed on a robotic platform is exposed to the environment much longer than the first plate.
Depending on the liquid matrix of the sample and the environmental conditions, liquid samples can decrease in volume through evaporation or increase in volume through absorption of atmospheric water. Such volume changes can affect the results of an experiment by changing the concentration of the drug candidate in an unexpected fashion. If absorption and/or evaporation is studied and understood, a scientist can take necessary precautions to reduce volume and concentration errors due to the laboratory environment.
Volume Measurement Technology
A quick and easy way to understand the severity of absorption and evaporation in plates is to monitor small volume changes over the course of a normal day using the VMS™ (Volume Measurement System) from Artel. The VMS quickly reports individual volumes of sample in each well of 96- or 384-well plates. Based on the ideal gas law, this technology measures the pressure of a known volume of air injected into the sealed wells of a plate, and calculates the well volume occupied by liquid or solid samples.
Application of Technology
To demonstrate the VMS technology, an experiment was conducted to measure volume changes due to environmental effects, using two 384 well plates (Greiner 781201). Plate 1 and Plate 2 were each filled with 50 µL of Dimethyl Sulfoxide (DMSO, Sigma Aldrich 276855) in columns 1–12 and 50 µL of tissue culture grade water (Sigma W3500) in columns 13–24 using a calibrated hand pipettor. The initial starting volumes of every well in each plate were measured by the VMS and recorded as Time Point 0. Both plates were then left on the lab bench and measured at the specified time intervals.
Plate 1 was left open to the environment while Plate 2 was protected using a plastic cover (VWR 28317-488). Every hour the volume in each well of each plate was measured using the VMS (Time Point 1 through Time Point 8). The cover on Plate 2 was only removed briefly each hour to take measurements. While the temperature and humidity of the laboratory were not controlled for this experiment, they were recorded for each time point.
Table 1 summarizes the results of the experiment. The volumes reported at each time point represent the average volume change, which corresponds to the average amount of water either absorbed or evaporated from each well at a given time point compared to the starting volume. Positive values represent a gain of volume while negative values represent a loss of volume. The starting volume of each well was approximately 50 µL.
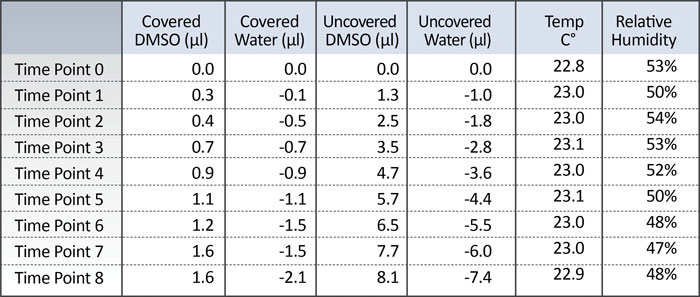
Table 1. Measured volume change
Measurable Effects
Using the Artel VMS, small changes in volume were measured over the course of the 8-hour experiment as illustrated in Figure 1. The water-filled wells in the covered plate lost an average of 4.2%, while the uncovered side lost 14.8%. The covered, DMSO-filled side gained 3.2%, and the uncovered, DMSO-filled side gained 16.2%, due to the hygroscopic nature of the solvent. There were also two other observations worth noting.
The first is an obvious “edge-effect” on each side of the uncovered plate. More water was lost around the edge wells than in the center—over 2%. The second observation involved the interface columns between the DMSO and water-filled wells (columns 12 and 13). Average water gain in column 12 (DMSO) was 3% greater than the mean average of the entire DMSO side, while average water loss in column 13 (water) was 5% greater than the mean average of the entire water side.

Figure 1. Change in measure volume over time
Interestingly, this effect was even more pronounced in the covered plate. Column 12 (DMSO) lost 9% more volume while column 13 (water) gained 9% more volume than the mean averages of their respective sides. These observations are illustrated in Figure 2. The proximity of the water to the DMSO appears to increase evaporation and absorption. The plastic cover used during the experiment did not produce the airtight seal that a heat or adhesive seal provides. Instead, the plastic cover seemed to provide a microenvironment wherein the DMSO in column 12 was able to act as a desiccating chamber for the water-filled wells in column 13.
Some condensation formed on the underside of the cover over the entire water section, but seemed to be more prevalent over the two middle columns. This also seems to show that the proximity of the hygroscopic DMSO in column 12 to the water in column 13 affected the evaporation of the water. The evaporated water did not all end up being absorbed by the DMSO; some also condensed on the cover.
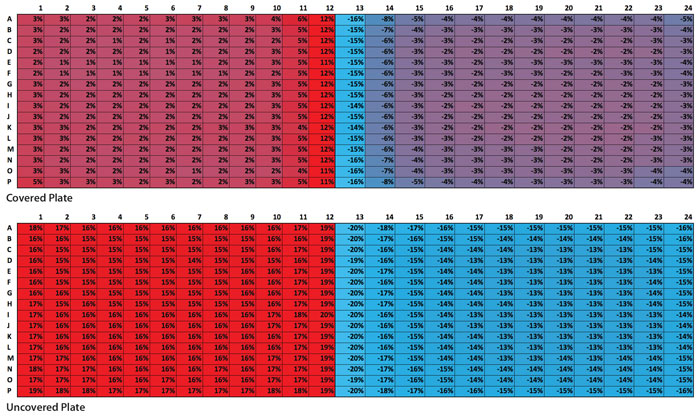
Figure 2. Heat map of measure volume change
The Effect of Environment on Assay Results
The laboratory environment can be a significant source of error when dealing with volume sensitive samples. Unfortunately, it is likely that this fact is commonly overlooked. Evaporation in aqueous solutions due to dry conditions, or absorption in DMSO solutions due to humid conditions can both cause significant changes in sample concentration. Almost all assays performed within microplates are concentration-dependent, and microplate wells that have higher/lower volumes due to water uptake/loss will result in unknown compound concentrations. If you do not know the concentration of your compound, you cannot draw an accurate conclusion about its activity or potency.
Assuming the laboratory conditions of the experiment reported herein, a starting aqueous solution of 10 mM would experience enough evaporation to result in a final concentration of over 12 mM by the end of the day if left open to the environment. Samples stored in solvents that are hygroscopic (such as DMSO) can also absorb moisture from the air, causing a decrease in the final concentration which can lead to poor results.
Controlling and monitoring the laboratory environment is one way to mitigate these risks. For instance, a sample storage laboratory that maintains collections stored in DMSO should have a dry, stable environment. Plate covers, adhesive seals, and heat seals can also reduce environmental effects. However, since these are not always feasible when using robotic systems, proper protocol design is essential. Even environmental modifications such as an inert gas (Argon) blanket can be extremely useful when plates need to be exposed for long periods of time. As shown by the experimental results, a lot can happen in 8 hours—especially if precautions are not taken to protect your samples. Understanding the rate of evaporation/absorption is key to developing a strategy for protecting sample integrity.
Bill Gigante is product manager/mechanical engineer at Stratec, and Tanya Knaide ([email protected]) is product manager and John Thomas Bradshaw, Ph.D., is senior development scientist at Artel.







