September 1, 2009 (Vol. 29, No. 15)
Nina Flanagan
Greater Emphasis on Early and Better Testing Pushes Molecular Diagnostics to the Forefront
Molecular diagnostic advances are being driven by the need for automation and easy-to-handle techniques, said Mark Bunger, research director, biosciences at Lux Research. Longer-term trends also driving this market include the influx of smaller companies developing diagnostic tools and a greater focus on end markets due to direct-to-consumer marketing.
The biggest hurdles, according to Bunger who spoke at “Molecular Diagnostics World” held last month in San Francisco, reside in validation. “Molecular tests based on the presence or absence of a protein or allele are the low-hanging fruit,” he said. “More complex conditions that are subtle or impossible to discern from molecular data alone have to be correlated with clinical data. These are poorly validated now, which is holding things back.” He added that pharmaceutical companies are focusing on companion diagnostics, “a new area that will help them refine and optimize drug sales.”
Bunger believes diagnostics will be pushed to the forefront with more emphasis on early and better testing, especially now with Dr. Francis Collins’ recent appointment as NIH director. “I think the field is poised for a great future,” noted Bunger.
He’s not alone in that assessment: the global market is estimated to reach $6.35 billion by 2015 according to Global Industry Analysts.
The push to commercialize simple and cost-effective molecular tests for second-tier clinical labs is the incentive behind BioHelix’ IsoAmp® assays. “Our mission is to improve the quality of healthcare through development of simple molecular diagnostic tests for the near-patient setting, where rapid solutions are necessary for prompt medical intervention,” stated Huimin Kong, Ph.D., CSO. By combining its isothermal helicase-dependent amplification technology with an instrument-free detection device, these assays fulfill the company’s objective.
“Currently, only top-tier clinical labs (approximately 600 in the U.S.) perform molecular diagnostic tests, while there are about 5,000 to 6,000 second-tier labs that don’t due to high costs and complexity,” Dr. Kong explained. The IsoAmp Molecular Analyzer platform is targeted at these labs. With this product, a helicase enzyme unwinds DNA into single strands, eliminating the need for a thermocycler and providing a method for assay development, Dr. Kong said.
Amplicon detection is achieved via the BESt™ (biohelix express strip) cassette. This is an enclosed, disposable cassette that is cross-contamination proof, and available to detect a single amplicon (type I) or two amplicons (type II), reported Dr. Kong. Results are available in about ten minutes versus having a test done at a reference lab, which can take a few days, he added. “There is a need for conducting molecular diagnostics in near-patient settings or ultimately at the point of care.”
The company’s lead IsoAmp assay is for MRSA and is in beta testing. Additional assays in development include: Staphylococcus aureus, Clostridium difficile, Chlamydia trachomatis, Neisseia gohorrhoeae, herpes simplex virus, and HIV (funded by NIH). The assay can also be used to detect genetic mutations such as SNPs that cause Factor V Leiden thrombophilia, Dr. Kong noted.

Molecular diagnostics are used to capture genomic and proteomic expression patterns to create new screening and diagnostic tools, monitor the effectiveness of a particular therapy, and help predict a patient’s response to treatment.

BioHelix’ IsoAmp Molecular Analyzer is an instrument-free molecular diagnostic platform for IsoAmp assays.
Prostate Cancer
Although high levels of prostate-specific antigen (PSA) can provide evidence of cancer, its use as a screening tool is controversial as normal prostate cells also shed this antigen into the blood. Prostate cancer gene 3 (PCA3) is being touted as a promising biomarker for prostate cancer. Gen-Probe has developed a PCA3 test that is currently on the market in Europe, initial clinical trials are set to start this quarter in the U.S.
According to the company, this first-generation test is semiautomated and semiquantitative and provides good clinical data. An endpoint assay, the PCA3 test uses chemiluminescence via the company’s hybrid-protection assay technology. A specific DNA probe hybridizes with a nucleic-acid target to emit a chemiluminescent signal. This is followed by target capture and amplification.
A prototype of the second generation of this assay is being developed as a real-time assay, combining amplification and detection in one step. “You can measure the kinetics of the reaction and that’s how quantitation is achieved,” said Norman Nelson, Ph.D., director of biochemistry. Fluorescence also allows measuring multiple signals in one tube. It’s amenable to the types of probes needed with real-time assays. “These are typically self-reporting probes, which are homogenous in that the fluorescence is quenched in a molecule with a stem-loop structure in the absence of target, but is unquenched in the presence of target.”
The multiplex format is more convenient, uses less reagent, and provides faster results, Dr. Nelson reported, adding that the biggest challenge when multiplexing nucleic acids is interference between oligonucleotides needed to build the test. “We solved this with a universal tag approach that’s unique to our methodology that makes amplification cleaner, with less interference.”
This technology has the potential for use in other cancers and disease areas, as well as in other markets driven by nucleic acid molecular testing, Dr. Nelson said. “These tests are all about oligos, and that’s one of the things that’s so powerful about DNA chemistry—it’s exquisitely precise and to make it work for you, you have to design it properly.”
Collaborating with researchers at the University of Michigan, Metabolon has discovered a number of markers indicative of the aggressiveness of prostate cancer and involved in the transition of noninvasive cancer to invasive.
“One of these compounds is sarcosine, a small molecule that is a methyl glycine,” explained John Ryals, Ph.D., CEO. “Glycine is used within cells to monitor or buffer levels of ethyl-densyl methionine, the methyl donor for methylation of DNA and other methylation reactions. When that goes too high, glycine is converted to sarcosine, which is mechanistically involved in this transition”.
Researchers have found that sarcosine levels are substantially increased during progression to metastasis. This small molecule, and several other discovered markers, can be detected in urine to differentiate aggressive versus nonaggressive cancers. “That’s the holy grail of prostate cancer, because most prostate cancers are not aggressive and won’t metastasize,” added Dr. Ryals.
The company has a platform built around its Metabolyzer™ data processor, which converts raw mass spec data into biomarker data. The biggest challenge in developing biomarkers, said Dr. Ryals, is obtaining source samples from existing studies. “Most of the time the studies aren’t designed in ways most efficient for discovering biomarkers, so you’re always trying to bridge an existing study to get the markers you want. In addition, banked samples are not always taken and stored the same way, causing large variation in analysis.”
Membranes for Plasma Seperation
A new polymeric, 3-D membrane developed by Pall Life Sciences provides one-step plasma separation from whole blood for use in downstream diagnostic assays. The asymmetric structure of the membrane captures cellular components without lysis. This is in contrast to the standard membrane (glass fiber), which often shears cells, leading to contamination. “Our membrane works with small volumes of blood [from 5 to 100 microliters] but results in full plasma recovery,” stated Galina Fomovska, Ph.D., senior principal scientist, molecular media R&D.
The membrane is available in three different grades, each optimized for various usage conditions, Dr. Fomovska said. GF is not treated and is for small blood applications, like finger sticks in microfluidic and lateral flow point-of-care devices. GX has low post-treatment to help minimize hemolysis and is also compatible with electrochemical analyte detection (good for up to 30 microliters). GR is for larger blood applications (up to 50 microliters) such as lateral flow immunochromatographic devices.
“We provide this separation membrane to people who are developing diagnostics—it’s designed for point-of-care use. We’re looking to move dependency away from the central lab and integrate our materials into a format that will allow testing for different analytes without having to centrifuge the plasma,” said Dr. Fomovska. She added that it is a “broad, universal enabler” that would also be of benefit for personalized medicine (e.g., glucose testing).
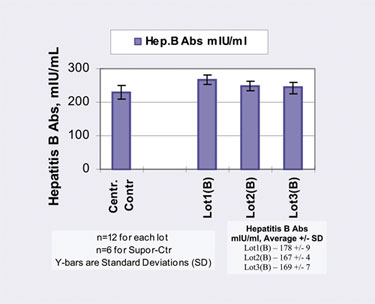
Pall Life Sciences’ Vivid™ Plasma Separation Membrane GF can be used for hepatitis B antibody recovery from whole blood.
Drug-Induced Toxicity Biomarkers
Drug-induced toxicity is currently measured by histopathology and blood tests, and the time lag for clinical diagnosis is problematic. Researchers at Compugen have developed a method to discover genetic biomarker signatures to predict the occurrence of drug-induced renal toxicity before it is detected by clinical chemistry, offering an opportunity for early prediction.
Animal studies using rats treated with well-known renal toxins found a subset of four to six genes that were selected as a biomarker signature, indicating nephrotoxicity at day five. The four biomarker combination identified the nephrotoxic drugs following a one- to five-day exposure period, as opposed to a typical 28-day diagnostic timeline, according to the company. In addition, Compugen scientists reported that the biomarker combination successfully predicted the relative levels of toxicity of the compounds tested.
“These results represent the first application of Compugen’s drug-induced toxicity biomarker discovery platform, which incorporates our rat-related predictive transcriptome and proteome,” explained Merav Beiman, Ph.D., head of molecular biology. He added that this database is substantially different in that the modeling of alternative splicing adds a large number of novel proteins and improves the quality of sequence prediction, contributing to the company’s ability to predict disease-related markers.
The platform is designed so that various components can be modified in order to use it for the discovery of novel preclinical biomarkers for other tissue toxicities such as cardio- or hepatotoxicities.







