March 15, 2009 (Vol. 29, No. 6)
Kathy Liszewski
Guidance on How to Do More with Less while Maintaining High Levels of Quality
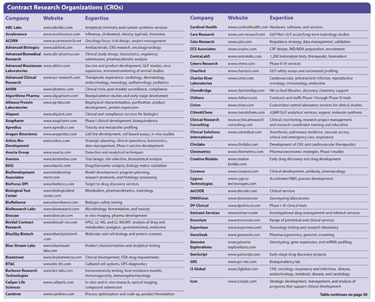
The pharmaceutical industry is, without a doubt, feeling the crunch of skyrocketing costs, lackluster pipelines, and drug safety issues. Clinical professionals need to do more with less, while still maintaining the highest levels of quality. Many companies are choosing contract research organizations (CROs) as a cost-effective strategy to outsource services ranging from drug development to managing and monitoring clinical trials.
The “18th Annual Partnerships with CROs and Other Outsourcing Providers” conference will be held next month in Orlando. The meeting will examine critical issues in clinical outsourcing partnerships such as improved methods of data integration, business partnering, and comparing global sites for clinical trials.
Biotech and pharmaceutical companies often choose CRO suppliers and the required central labs independent of each other. The ultimate goal of outsourcing is to achieve three project drivers: efficient use of time, high-quality products, and mutual value-based cost (i.e., the price charged is equivalent to the value of the service provided), according to Cathy Michael, executive director project and data management, global central labs, PPD. Separate providers, however, can only achieve two, and that has been traditionally considered acceptable.
“We feel it is possible to achieve all three goals by using a central lab already associated with a CRO. When data management is provided in this way there is more accurate communication among all parties and all sites. For example, Michael says, “there may be multiple times a day when data, protocols, and other information needs to be added to or accessed from the various databases held at the CRO, third-party labs, and central lab. About six months ago we developed an Oracle-based system that integrates and refreshes applicable data between our CRO and central lab.”
An important benefit of such integration is the ability to input patient data and visit it quickly via a common format that supports real-time data cleansing of patient data traditionally held in multiple repositories. “The need to reconcile at the end of a study is moot when you have one central repository allowing for a single reconciliation effort for lab and CRO.”
Michael has some advice for companies about to choose a CRO. “It is most important to look at how companies integrate databases and to develop a long-term relationship from beginning to end with how data is captured and processed. You’re more likely to avoid costly data-integration problems later, if you do this up front.”

MDS Pharma Services’ central lab operation in Beijing moved to a new facility a few years ago, tripling the size and increasing testing capacity fivefold over the previous location.
Worldwide Data Capture
Integrating data from many global clinical sites can be a real challenge, reports Ross Rothmeier, senior director, EDC Portfolio, Covance. “It can get complicated to combine data obtained from multiple sources in global clinical trials. But this is important because submissions require all the data collected, and it has to be in a consistent format to review.”
According to Rothmeier, it is particularly challenging to have straightforward and easy access to site and laboratory data coming from multiple locations—especially on a global scale. One solution is electronic data capture (EDC).
“If you have sites in 47 countries in a clinical trial and are using multiple labs,” explains Rothmeier, “it can be difficult to get an overall view of the data in the trial. Solutions requiring loading and converting data into a site’s computer, or collecting it on paper and then transcribing it into another system, are cumbersome and introduce many points of potential failure and error.
“EDC allows the entry of data into a central repository and uses edit checks during entry that help reduce errors. Whether entered at the investigator site or transmitted directly from a laboratory, EDC simplifies the process and increases data quality. The central repository makes this data available to anyone who has authorized access, regardless of location, and does so more quickly than paper or decentralized systems.”
Rothmeier adds that another challenge for the industry is adopting data-definition standards. “Many organizations are being very forward thinking in endorsing industry standards such as CDISC (Clinical Data Interchange Standards Consortium) and HL7 (Health Level 7) that provide specific guidelines for data interchange. This is important as it enables data from multiple sources to be combined without converting or transcribing it to a proprietary format. This further simplifies the process and reduces the challenges of conducting a global trial.”
Strategic Planning
When forming partnerships with CROs, companies often employ one of two approaches, transactional or strategic, says Daniel Spasic, CEO, Trial Form Support International (TFS).
According to Spasic, a transactional approach means, “that a company chooses to tactically outsource projects and assignments based on what is most suitable for the project. But this approach also suffers from not charting a clear pattern since it is based primarily on each individual project. Therefore, you can end up with several providers, problems with internal administrative coordination, and underutilized cost benefits.”
Spasic believes that using a strategic approach that employs a few selected providers makes more sense. “With a strategic approach you would choose one CRO for data management and a different CRO for clinical development work. This will be more efficient in the long run because it will save internal administration time and you will likely get better pricing and attention since the CRO you are outsourcing to can rely on more regular workflow.”
Another important issue to consider when selecting a CRO is where the company conducts its clinical trials and the extent of its geographical reach. “This depends on what is being outsourced and the development phase of the clinical trial. If a company is outsourcing early clinical development, that could be done locally with specialized units. Here you must look primarily for competence and experience, and what technical capabilities the unit has.
“But, if you do proof-of-concept studies with 100–250 subjects,” he continues, “then that can be regionalized to three or four countries in Europe, or only the U.S. Mid- to large-sized CROs are appropriate for these. If you are conducting pivotal Phase III trials, however, you need to do that on several continents and there you’d select bigger CROs with the possibility to support you through its own global infrastructure.”
Spasic expects lively discussions at the Orlando meeting as to which countries are the best for outsourcing. “There are major concerns that the U.S. will lose out on trials because federal regulations and lack of patient interest slow down the process. But wherever a trial is conducted, including China and India, each place will have unique advantages as well as disadvantages. There is no one right place.”
TFS currently has approximately 200 clinical studies of which 15% are in the U.S.
Trials in China
Performing clinical trials in China also has both advantages and disadvantages, according to Patrice Hugo, Ph.D., vp, scientific affairs, MDS Pharma Services.
“The differences in Chinese trials and in global programs that include China are not unique to a geography or therapeutic specialty. As with any country, there are processes, regulations, and culturally specific issues that affect the timeline and resources needed to conduct a trial.
“Issues that affect a trial’s timeline include the acquisition of a clinical trial permit. Unfortunately, this process could delay study start time significantly, up to a year from initiation. Additionally, there are regulatory limitations in China on the exportation of matrices, which limit the analysis of these specimens to in-country resources.
“Having regional contacts and affiliations may expedite the process. However, there is no substitute for local presence and expertise. A detailed understanding of the regulations and available options is critical to expediting study start-up, remaining on schedule, and proper resource forecasting.”
According to Dr. Hugo, in most instances, trials planned in China have initiation and forecasting challenges resulting in start-up delays, but quick enrollment for patients, which accelerates the timeline comparative to other geographies. “This becomes an issue for long-term treatment protocols as the program milestone curve is compressed and may pose a challenge to underexperienced teams managing global programs that include China.”
Finally, Dr. Hugo suggests that “many regional resources may have the appropriate technology, but lack experience in global project management. Or, they may have limited systems to ensure the consistency and control of the chain of specimen custody. Keeping a critical eye on these factors is key to the success of your programs.”
Thinking outside the traditional CRO concept is the goal driving ReSearch Pharmaceutical Services. About 10 years ago, the company decided to “build the industry’s first next-generation CRO,” according to Samir Shah, vp, strategic development.
“Our integrated business model differentiates us through our ability to deliver maximum flexibility,” Shah notes, “offering both solutions that are fully integrated with our client’s infrastructure and those that encompass more traditional outsourcing programs,” Shah notes.
According to Shah, the company’s “fully integrated solutions allow clients to outsource those portions of the clinical development process for which the greatest efficiencies and savings can be realized, while maintaining strategic control over key medical and regulatory functions. This close collaboration means that our solutions are not limited to just one client project or protocol, but can cover the entire breadth of our clients’ drug development pipeline.
“By embedding ourselves within our clients’ infrastructure, we create a strategic and interdependent relationship which allows us to anticipate our clients’ clinical trial demands and efficiently deploy our management and clinical team to meet the clients’ needs.”
Shah suggests that more medium to large biopharmaceutical companies are moving toward this partnering model. “We feel that the service provider landscape, mainly traditional CROs, have to change their business model in order to be more adaptable and flexible. While the greatest growth for traditional CROs in the long term will come from smaller, virtual organizations that do not have the ability to conduct clinical trial work, CROs will have challenges meeting the needs of large- and medium-sized biopharmaceutical firms. As the industry continues to consolidate, the traditional CRO model will have impact in terms of continued growth and associated profitability.”
Adaptative Trial Innovations
Adaptive clinical trials are nothing new, but new innovations in trial design and implementation are helping to make it a more efficient and cost-saving process, according to Imogene Grimes, Ph.D., vp, data sciences strategic services, Parexel.
“The biggest challenge is getting the clinical trial protocol right the first time,” Dr. Grimes says. “Even if the execution is perfect, it’s no good if the design is wrong. An adaptive clinical trial is one in which there is a modification in the study based upon generated information. The necessary change could be in sample size, drug dosing, randomizing, or when there is more than one study under the same protocol.
“A traditional saying is, ‘plan your work and work your plan.’ However, when we looked at different scenarios to see how we could change adaptive trial design to make it more efficient, we found that it was possible to combine Phase II and Phase III under the same protocol.”
According to Dr. Grimes this innovation encompasses three major aspects. “First, you can consider reusing patients in Phase II and Phase III trials. A second dimension is to determine if you can make a decision to go with 90% of the data as opposed to 100% of the data from Phase III. Third, companies should evaluate if they have an appropriate penalty scenario built into the trial design.
“The term alpha refers to a variable describing whether a drug works or not. Of most concern is a decision that it does work, when actually it doesn’t. Typically, the FDA allows 5% for alpha. But you must spend this wisely as error will go up unless you impose safety measures. We found you can optimize this aspect by making projections about how many patients need to be enrolled, for how long, and the resulting cost. The bottom line is that it’s important to develop protocols with and without a penalty.”
The CRO future is bright, opines Dr. Grimes. “This is an exciting time for CROs, especially because of new and amazing technologies, a change in the regulatory attitude, and because more guidance is available now than ever before. The CRO, sponsor, and regulatory agency all must share in the process of bringing a new product to the patient.”
Advice from the Experts
- Do not underestimate the importance of database integration. It is critical to work closely with your CRO from the beginning on how data is captured and processed.
- Electronic data capture provides straightforward and easy access to site and laboratory data coming from multiple locations.
- Often it is most advantageous to choose one CRO for data management and a different CRO for clinical development work. This could be more efficient as you will likely get better pricing and attention since the CROs to which you are outsourcing can rely on more regular workflow.
- Having regional contacts and affiliations may expedite the process if you are outsourcing to another country, however, there is no substitute for local presence and expertise.
- Sometimes it is possible to combine Phase II and Phase III under the same protocol.







