August 1, 2009 (Vol. 29, No. 14)
Angelo DePalma Ph.D. Writer GEN
Getting It Right Involves More Than Simply Multiplying Vessel Size and Equipment
Disposable bioprocessing, platform expression and purification technologies, and a higher level of bioprocess understanding have made scale-up more robust and predictable, but not quite automatic.
Communication between lab and pilot-scale personnel is critical for successful scale-up, says Klaus Graumann, Ph.D., head of technical development at Sandoz. The company’s Kundl, Austria, production site uses microbial expression systems to produce cytokines, antibody fragments, and peptides. It also performs development for other products and biosimilars.
Lab and pilot groups should work closely, interact, and communicate early in the process to minimize scale-up problems. Co-location of the two competencies can reap huge rewards for understanding and meeting the differences between development and production. “The key to success is to eliminate as many failures and disconnects as possible,” he says.
Dr. Graumann stresses the value of experience and the adoption of platform technologies whenever possible. Processors may still encounter hurdles when utilizing optimized cell lines, media, and unit operations, “but at least they know where to start” to look for problems if issues arise, he notes.
Products introduced into a plant for the first time are particularly prone to scale-up issues, since few production plants are running product for which they were designed. Questions of process–plant fit are common. “Matching process to plant is not much of an issue in early development, but it’s important for laying the foundation for cost-effective process development,” says Dr. Graumann. “Processors prefer not to have to go through too many changes since they introduce comparability questions and regulatory scrutiny.”
The Role of Outsorcing
Contract manufacturing has become a major factor in scale-up work, as it is for manufacturing in general. Virtual companies or firms with minimal production capacity rely on contract manufacturing organizations (CMOs) to perform the heavy lifting of scaling from spinner flasks to the several hundred-liter scale. At this stage in a product’s lifecycle, time is of the essence since preclinical studies cannot begin without sufficient material.
CMOs like Avid Bioservices will often screen for factors that increase production at small scale, then quickly transfer those conditions to small bioreactors. “It comes down to modeling the large process on a small system, showing comparability across scales, and reaching an understanding of variability as the scale increases,” says Thomas Tomzynski, supervisor of upstream process development.
That level of understanding is impossible to achieve in a timely manner without process analytics, real-time measurement of nutrient utilization, and waste product generation, says Tomzynski. “We try to characterize the process as much as possible moving through scale-up, to achieve acceptable product titers and quality.”
Clients normally transfer processes to Avid early, sometimes as soon as cell lines are optimized. After selecting a clone, Avid determines baseline and selects conditions that optimize area under the growth curve, one of the best indicators of productivity. Since speed is highly valued, Avid relies mostly on off-the-shelf media and feeds to optimize the process in the shortest time.
“Off-the-shelf media don’t require a lot of custom tweaking,” observes Tomzynski. “We can try several of them rapidly, and scale them quickly as well.” Avid relies on HyClone disposable bioreactors from Thermo Fisher Scientific for 100 L runs but also uses 5 L and 36 L glass bioreactors and a 1,000 L stainless steel reactor.
The goal is to reach GMP manufacturing rapidly so clients can submit their INDs, and continue supporting clinical and commercial manufacturing.
Tomzynski notes several hurdles to scale-up, notably carbon dioxide build-up at larger scales, pressure on downstream operations due to high titers, and technology transfer. Avid addresses the first with technology to reduce CO2 in both stainless steel and disposable bioreactors.
The downstream issue involves mostly monoclonal antibodies and the capture step, which is conducted with protein A. Tech transfer is another perennial problem as customers often have characterized processes in less-than-ideal fashion. “They don’t always bring in as much information as we would prefer,” says Tomzynski.
Scale-up-based contract relationships are often built around specialized expertise or proprietary technology, for example, formulation, experience with a particular cell line or dosage form, or quality track record, notes Barath Subramanian, senior industry analyst for pharmaceuticals and biotechnology for Frost & Sullivan. “Quality advantages provided by the contract manufacturer are then transferred into the product as part of the outsourcing relationship.”
He mentions Patheon as a CMO offering development and scale-up services for small molecules and Catalent Pharma Solutions for both drugs and biologics.
The economic downturn negatively affected demand for scale-up services from both small and large biopharma, Subramanian adds. Quite a number of small- and mid-sized firms, including discovery-stage companies that outsource most of their development work, have disappeared while others have merged, thus reducing the number of bench-to-development and development-to-pilot scale-up projects.
Even larger companies have scaled back on early-stage molecule development, he says. This slowdown will, five or so years down the road, reduce demand for development and scale-up services from CMOs. Subramanian predicts slower growth for these companies as a result. He does not believe the slowdown will be catastrophic, however, since CMOs can live off the growth from the last decade. “Even with this short-term setback CMOs should be able to maintain growth in the high-single to low-double digits.”
Might of the Unliving Dead (Cells)
Cell-free protein synthesis has been one of the more interesting options for protein production, but the method suffered from a lack of scalability. That is rapidly changing.
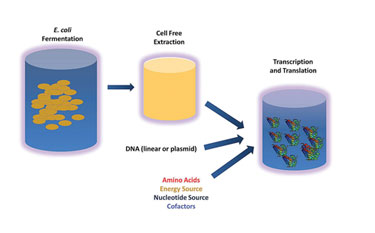
Research biologists routinely use cell-free preps to generate small quantities of protein for experimentation and characterization. Sutro Biopharma, which recently licensed cell-free intellectual property from James Swartz, Ph.D., of the chemical engineering department at Stanford, has engineered the technology to permit cell-free scale-up to thousands of liters and beyond.
The process essentially hijacks the protein-producing mechanism of nonliving E. coli organisms. Organisms are lysed and minimally treated to preserve transcription and translation capability, then frozen for storage and shipping.
Sutro employs glucose, glutamate, and pyruvate as carbon sources for proteins and nucleotide monophosphates for genes. Energy is derived from nucleoside monophosphates, which are regenerated in situ, rather than more expensive triphosphates. The instructions for protein or gene construction are contained in a plasmid, with a polymerase added for propagating the gene.
Sutro achieves g/L volumetric productivity in a 10-hour reaction (as opposed to a 7–10 day cell culture). Products produced through this method include cytokines (for example G-CSF and GM-CSF), antibody fragments, Fc fusions, and enzymes. “We haven’t exhausted the possibilities yet,” says Dan Gold, Ph.D., president and COO.
The company is currently working at the 100 L scale but there is no theoretical or practical reason why it cannot be scaled to 1,000 L or more. This volume, says Dr. Gold, could service demand for almost any therapeutic protein since the process can be repeated several times per week. A 1,000 L reaction would require extract harvested from a 2,000 L E. coli fermentation. The only limitation, which might eventually be overcome by coupling cell-free synthesis with other biotransformations, is that E. coli lack the machinery to glycosylate proteins.
What is remarkable is that Sutro achieves nearly seamless scalability without changing cell lines or reformatting, and with practically no process development in the traditional sense as one would expect with growing cells and microorganisms.
The process advantages for cell-free systems in large-scale protein production are potentially huge. Unit operations used in cell-free synthesis are scalable and familiar to those who work on microbial fermentation—mostly filtration and centrifugation. A single extract is suitable for production of any protein. And, unlike living cells, extracts are available for use on short notice. Since the E. coli extracts are “instructed” to produce only one protein, host cell protein issues generally disappear. Purer product streams mean leaner downstream separations as well.
“We believe that transforming biological reactions into a controllable enzymatic synthesis will revolutionize the way people look at protein manufacturing,” says Dr. Gold.
Sutro Biopharma’s cell-free synthesis technology process flow
Validation—The Critical Link
Thorough validation of scale-up strategies can save major headaches as the process is built up and out. The point is to demonstrate that products manufactured at large scale for market distribution are chemically and biologically equivalent to those produced at small scale for preclinical and early-stage safety testing.
In biomanufacturing the process is the product, as Genzyme learned with Myozyme/ Lumizyme treatment for Pompe disease. U.S. regulators ruled that the product produced at 2,000 L scale was glycosylated significantly differently than the material manufactured in 160 L batches. “Scale-up is a risk,” observes Harald Dinter, Ph.D., vp of global biological development at Bayer HealthCare. “Whenever you change the process, even if only minimally, there is the risk of changing the product.”
Bayer’s Berkeley, CA, facility manufactures clotting factors for hemophilia, while its Wuppertal, Germany, site produces monoclonal antibodies and other biologicals.
Normally a bioprocessor will validate a large-scale process through scale-down studies, where the bioreactors, clarification, chromatography columns, and filtration systems are precise scaled-down versions of the production-scale processes. Not every process enjoys that luxury. For example, Bayer could not produce a reliable scale-down model for producing Factor 8, a large, complex, highly glycosylated molecule in perfusion reactors, so it performed development work at full commercial scale. “It was obviously costly to do,” notes Dr. Dinter, “but we felt we needed to do that to assure the safety of the product.” Purification studies were performed in scale-down mode, however.
Factor 8 is perhaps an extreme example compared with monoclonal antibodies, since it is larger and carries more glycosylation sites. The potential variations are, therefore, greater than with antibodies and warrant a two-track development model for expression and purification.
Validation is somewhat easier when platform manufacturing technologies are available, which is common with mAbs. Companies that specialize in antibodies often boast of cell culture, harvest, and purification that follows an almost identical path from product to product.
Disposable bioprocess equipment is likely to present novel validation problems as processes progress from 500 and 1,000 L bags to 2,000 L stainless steel vessels and beyond. Plastic and steel appear to produce equivalent products, as studies at Bayer and other firms suggest, but the number of molecules compared in this fashion is still relatively small. The plastic-steel issue will probably not last long, however. As Dr. Dinter points out, cell culture titers are rising rapidly enough that some products in development today could eventually be manufactured in plastic vessels of 1,000–3,000 L.
Biomanufacturers have numerous options for scale-up validation support. Charles River Laboratories provides analytical support for scaling from master cell bank through full production. While processing occurs at the sponsor’s site or at a CMO, Charles River becomes the eyes and ears for scale-up validation, says Barry Rosenblatt, Ph.D., director of biopharmaceutical technology development. The company examines product attributes such as identity, safety, potency, chemical characterization, and virologic profile at every scale-up stage, looking for biological or physiochemical changes that might affect safety.
The most common discrepancy between scales, according to Dr. Rosenblatt, are in post-translational modifications. For mAbs this includes, but is not limited to, glycosylation, a factor critical in some antibodies for biodistribution, potency, and immunogenicity. Post-translational changes such as glycosylation and deamidation, which affects a protein’s charge profile, commonly occur as a result of changes in the cells’ housekeeping functions. Other common differences include changes in impurity aggregation behavior and impurity profile.
“The easiest mistake to make is to assume that if it works at the bench, then scale-up involves nothing more than multiplying the sizes of vessels and equipment,” says Dr. Rosenblatt. Maintaining identical conditions for mixing and column chromatography, for example, require a good deal of engineering, he observes. Scale-down models, when available, are useful for predicting how larger processes will proceed, but to validate a scale-up requires “thinking in both directions, scale-up and scale-down,” he says.

Bayer HealthCare’s Berkeley, California, plant manufactures clotting factors for hemophilia.

Characterization of the product and process are key elements in the successful implementation of process scaling, according to Charles River Laboratories.







