May 15, 2010 (Vol. 30, No. 10)
Blake D. Anson Ph.D. Product Manager Cellular Dynamics International
Stem Cell Technology Can Facilitate Quick & Early Failure of Toxic or Ineffective NCEs
One axiom of the pharmaceutical industry is to “fail fast and fail early,” thereby minimizing investment in toxic or ineffective compounds. A primary hurdle to realizing this adage is the use of cell models, primarily cell cultures derived from nonhuman animals or immortal cell lines derived from tumors, which do not fully recapitulate principal in vivo functions.
The identification and isolation of human embryonic and induced pluripotent stem cells (iPSCs), along with techniques for guiding their subsequent differentiation into terminal cell types, allows the use of human cells as a more relevant cell model to efficiently drive discovery and toxicity assessments. Additionally, because iPSCs can be derived from individuals with identifiable phenotypes and genotypes, targeted human subpopulation models can be employed early in the discovery and toxicity screening processes.
The pharmaceutical industry requires large numbers of purified cell types for screening candidate molecules for efficacy and unintentional toxicity, and the industrialized use of terminal cell types derived from iPSCs has been severely hampered, if not prohibited, by the difficulties of culturing stem cells.
iPSCs, while highly proliferative, are sensitive to manipulation; improper handling can severely restrict their pluripotency and drastically reduce the numbers of subsequently differentiated healthy cells.
Furthermore, while producing terminally differentiated cell types from stem cells using embryoid body (EB) and directed differentiation techniques are well known, the efficiency with which these methods produce terminally differentiated cells is highly variable; a common theme to both techniques is difficulty in producing highly pure (>90%) populations of terminally differentiated cells.
Therefore, the key to utilizing stem cell technology on an industrial scale is to develop processes that are both scalable and standardizable for both iPSC maintenance and differentiation.
Cellular Dynamics International’s (CDI) iCell™ Cardiomyocytes are human iPSC-derived cardiomyocytes that possess expected cardiac characteristics, form electrically connected syncytial layers, and exhibit expected electrophysiological and biochemical responses upon exposure to exogenous agents.
CDI’s new technology overcomes barriers in both iPSC maintenance, terminal cell type differentiation, and purification by generating standardized and scalable protocols. The primary production constraint of iPSC husbandry was eliminated by developing a culture system that uses standard single-cell splitting techniques and small molecules to minimize operator-specific effects.
iPSC culture scalability was incorporated into the process by building the cell culture system in a parallel fashion to enable the production of billions of iPSCs through the use of CellSTACK® culture chambers (Corning).
Differentiation of iPSCs into iCell Cardiomyocytes is built on CDI’s platform that utilizes recombinant genetic engineering and antibiotic selection. Prior to iPSC clonal expansion, genes encoding antibiotic resistance and an optional marker under control of a cell-type specific promoter (pan-cardiac for iCell Cardiomyocytes) are introduced into the iPSCs through homologous recombination.
After curation and quality control (QC), the iPSC clone carrying the selectable marker is expanded using iPSC maintenance procedures, harvested, and placed into the directed differentiation protocol of choice. Subsequent to differentiation initiation, the cultures are exposed to the selection agent to leave a pure, targeted cell population.
In the case of iCell Cardiomyocytes, the directed differentiation method produces cardiomyocyte purities greater than 50%, while antibiotic selection subsequently increases this purity to approximately 100%, a level that is necessary to ensure that the observed experimental outcome is due to an effect on cardiomyocytes rather than noncardiac “contaminating” cells.
This process, as currently practiced at CDI, is capable of meeting the foreseeable demand for purified iPSC-derived human cardiomyocytes and is scalable by more than two orders of magnitude, without difficulty, if necessary.
Electrophysiological and Biochemical Responses Mimic In Vivo Cells
Although purified iPSC-derived cardiomyocytes have the physical appearance of cardiomyocytes and aggregations of the cells exhibit synchronous, syncytial, contractile activity (i.e., the cells beat), it was necessary to test the cells’ electrophysiological and biochemical responses to antagonists to determine their utility for drug development and toxicity testing.
Cardiomyocyte subtypes of the heart have distinctive electrophysiological profiles that can be characterized by, among other items, early depolarization events (phase 4 depolarization) and the duration of the depolarized plateau potential. Action potentials produced by individual iCell Cardiomyocytes recapitulate the action potentials of native nodal, atrial, and ventricular cardiomyocytes.
iCell Cardiomyocytes also mimic typical in vivo responses to electrophysiological antagonists. Electrophysiological responses of cardiomyocytes were tested against exposure to E-4031 (Figure 1), a well known blocker of the human cardiac Ether-a-go-go related gene (hERG) potassium channels. Just as hERG channel block prolongs cardiac repolarization in vivo, E-4031 application prolongs, in a dose-dependent manner, the field potential duration generated by iCell Cardiomyocytes as measured on a microelectrode array platform.
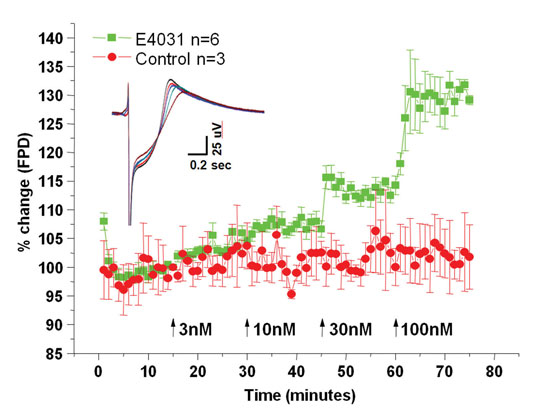
Figure 1. Prolongation of the iCell Cardiomyocytes field potential duration in response to increasing concentrations of the hERG channel antagonist E-4031 was recorded using MEA platform technology. Y-axis is percent change in field potential duration relative to control conditions, while the x-axis illustrates experimental time. E-4031 additions are illustrated by the arrows, and the inset shows representative field potential waveforms during each application from control to 100 nM.
iCell Cardiomyocytes also show sensitivity to known cardiotoxic compounds affecting biochemical processes (Figure 2). Cardiomyocytes were exposed to a diverse set of compounds known to have adverse effects on cardiomyocytes over a broad range of EC50 values. Cell viability was subsequently assessed via CellTiter Glo (data courtesy of Chad Zimprich, Promega) and demonstrated an expected sensitivity.
Cell handling plays a critical role in cell-based assay investigations. iCell Cardiomyocytes, like terminally differentiated native cardiomyocytes, do not divide in culture. Thus, mistakes in plating cannot be undone by simply waiting for the cells to divide and/or splitting and replating. Therefore, cell viability upon thaw from cryopreservation and the plating efficiency, i.e., the ratio of cells added (seeded) into a well over the actual number of cells that attach to the bottom of the well, must be taken into consideration when setting up experiments.
In the case of iCell Cardiomyocytes, an investigator can either use these two metrics, which are part of the QC program, as supplied by CDI, or they can determine cell viability on their own and combine that result with the supplied QC plating efficiency to determine the appropriate number of cells to place in each well.
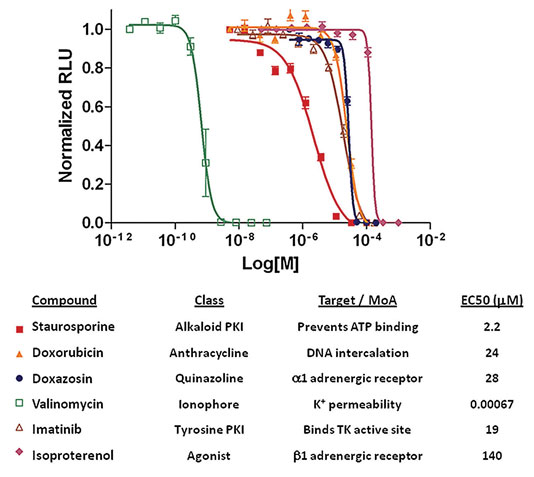
Figure 2. iCell Cardiomyocytes viability in response to antagonist application: Cell viability in response to application of known cardiotoxicants was assayed with Cell-TiterGlo (Promega). Y-axis is relative luminescence units, while the x-axis is cardiotoxicant concentration.
Summary
Cellular Dynamics has developed a highly standardized, scalable process to manufacture human iPSC-derived cardiomyocytes. Using this process, we can produce the industrialized quantities of human cardiomyocytes needed by the pharmaceutical industry for drug toxicity testing.
These cardiomyocytes demonstrate typical physiological properties and show appropriate sensitivity to cardiotoxic compounds expected of human cardiomyocytes. The ability to consistently produce large numbers of high-quality and high-purity cardiomyocytes offers the pharmaceutical industry a new tool to better predict the cardiotoxicity of new drug candidates.
It is expected that these cells will allow pharmaceutical companies to more efficiently select drug candidates while reducing the likelihood that cardiotoxic activity of these compounds will manifest themselves late in development or after regulatory approval and market launch.
Blake Anson, Ph.D. ([email protected]), is cardiomyocyte product manager at Cellular Dynamics International.







