February 1, 2010 (Vol. 30, No. 3)
Nina Flanagan
Technological Advances Provide Benefits in Drug Discovery, Dosing, and Epigenetics
In vivo imaging technology is advancing rapidly and expanding to include not only drug discovery and development but also diagnostics. Current efforts range from developing novel brain-imaging agents to further understanding the molecular basis of human behavior to engineering antibody fragments for cancer imaging biomarkers.
There is also a growing trend to combine imaging modalities, such as MRI with PET or PET with CT, allowing for additional studies. Furthermore, pharmaceutical companies are working to develop imaging agents with therapeutics. CHI’s “High Content Analysis” conference and Molecular Medicine’s “Tri Conference” both provide a glimpse into the future of this field and the promise it holds to provide a better understanding of disease.
Although in vivo imaging continues to evolve rapidly, it still harbors challenges. “The ability to see within live animals or humans with these technologies is very powerful. Driving toward real applications for studying disease or monitoring drug therapies is costly and the science and technology intensive,” stated Matt Silva, Ph.D., head of the imaging group at Millenium: The Takeda Oncology Company.
Dr. Silva is developing assays that are sensitive and accurately read-out both disease and responses to drug therapy for preclinical and translational imaging. Efforts also include evaluating new technology with the goal of developing robust applications to benefit internal research.
One of the imaging procedures that his group is currently working with is dynamic contrast enhanced magnetic resonance. This is a method to monitor blood flow and permeability within a tumor, and it is used with drugs that target vasculature.
“You can assess very early whether a patient is responding to treatment. For early-phase clinical trials, it provides information on whether the mechanism of action that’s expected results in a functional change in the tumor. For novel targets, that’s a very critical question,” he said.
In a normal experiment using this method, a scan provides a static image—with no information about how the signal changed as a function of time. According to Dr. Silva, his group has been performing kinetic analysis via rapid scanning and then extracting from the data hemodynamic parameters that reflect the microenvironment of the tumor. These changes are monitored when administering therapy. This has been shown to work in highly perfused tumors like breast tumors.
A potential challenge with this method includes signal to noise—there has to be enough contrast agent to penetrate tissues for an accurate model of perfusion. “Since tumors are heterogeneous, imaging is one of the only ways to model that heterogeneity.”
Another key area of research involves inflammation and oncology. Bone topology analysis provides information on bone erosion during disease (arthritis) and bone cancer. “We created an algorithm in 3-D that allows us to visualize bone erosion and to quantify it. What we’re trying to do preclinically is to demonstrate that an internal drug is having an effect on this model.”
According to Dr. Silva, his team has also used this algorithm to study dose and scheduling as well as provide comparison against control drugs. Another potential application is to analyze tumor structure. “We’re not sure whether this matters, but the way cancer invades tissues may be related to malignancy.” Overall, Dr. Silva summarized that his group is doing preclinical research to see if any of these imaging techniques will add value in early clinical trials. They are also focusing on molecular biology to create probes to help understand more about tumor microenvironments.
Tracking Transplanted Cells
Recent progress in cell therapy has increased demand for real-time detection of transplanted cells, which provides critical information on cell behavior in vivo to determine optimal dosage and delivery routes. Celsense is addressing these demands with its fluorine-based imaging reagents (Cell-Sense, V-Sense) and imaging software (Voxel Tracker™).
“Our approach is MRI-based, doesn’t use any ionizing radiation, and provides good contrast with no depth-limitations,” explained Eric Ahrens, Ph.D., founder and CSO. This also allows for longitudinal studies days or weeks after administration of the imaging agent.
Over the past few decades, metal-ion based contrast agents have been used to tag cells. However, Dr. Ahrens says these probes lacked specificity, making it difficult to interpret where the cells were located, and often required prescans before and after cell implantation to examine differences in contrast in that region.
Cell-Sense uses Fluorine-19, which is nontoxic and readily enters a wide variety of cells in culture (including immune and stem cells) tagging them ex vivo without requiring transfection agents. The labeled cells are inoculated into the subject and imaged with MRI.
Images of the Fluorine-19 labeled cells and proton (background anatomy) channels are acquired in the same imaging session. A composite image is constructed using Voxel Tracker software, showing the regions containing the labeled cells. Since there is no fluorine in the body, this provides unambiguous detection. Cell-Sense is now manufactured under GMP conditions suitable for human use, and the company has opened a Drug Master File with the FDA for use in clinical trials.
Another key use for Cell-Sense is to allow the tagged cells to circulate and then assay the biodistribution without histology, which “is very often a bottleneck in preclinical studies,” according to Dr. Ahrens. A panel of tissues is run through a conventional liquid NMR, and the number of tagged cells in the tissue sample calculated.
“This is a high-throughput screening technique for visualizing biodistribution of the cells throughout the organism.” A dual-mode version of Cell-Sense is also available that can be detected by MRI and conventional fluorescence-based techniques such as confocal microscopy or flow cytometry. This is useful for validation studies.
V-Sense, a fluorine-based reagent for direct in situ labeling of monocytes and macrophages, enables visualization of hot spots of inflammation. Preclinical applications include autoimmune disease, cancer, coronary artery disease, infectious diseases, injury, and organ/tissue transplant rejection.
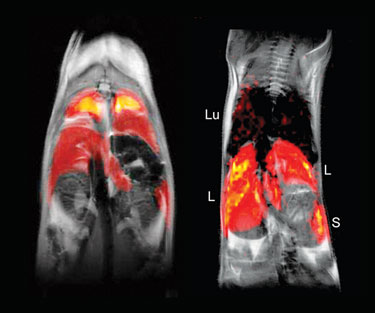
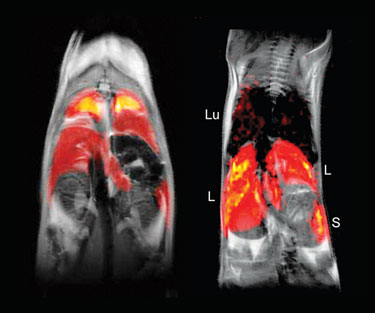
In vivo MRI of 19F-labeled dendritic cells in a mouse (Celsense)
Drug Action in the Brain
Researchers at Brookhaven National Laboratory are developing tracer molecules for imaging drugs in the brain. This has unique challenges, says Joanna Fowler, Ph.D., senior chemist, and director of the radiotracer chemistry, instrumentation and biological imaging program.
“Designing these molecules is one of the biggest challenges because we can’t accurately predict how a molecule is going to behave in a living system.” Another challenge is developing synthetic methods that work efficiently and rapidly for incorporating short-lived isotopes into these molecules. A third challenge is designing a drug molecule with a part that can be labeled in case there’s a need to know where the drug goes and where it binds.
“If you are developing a drug for binding to some glutamate transporter for depression, for example, think of putting a structural feature in the small molecule so you can eventually label it, like carbon-11 or fluorine. If you think this way when you are just developing a drug, there are many molecules that can be labeled.”
Dr. Fowler’s group has developed new methods to incorporate C-11 into organic compounds, increasing the potential number of structures available for labeling.
Her group has also worked with drug companies that want to know how much of a drug to administer in clinical trials. If the company has a drug that binds to dopamine receptors, and her group has a tracer for these receptors, it provides information on the occupancy of the receptors and how much drug to give and how often. Once a drug passes safety guidelines, the tracers, used with PET, can provide efficacy information. “You can measure concentrations of the drug in plasma and see if it correlates with what engages the target in the brain—if it does, you would have a biomarker.”
A growing area of research is focused on epigenetics. “We’re very interested in the enzymes that put epigenetic marks on DNA. There are enzymes that put methyl groups on DNA and shut down gene expression that are sometimes heritable. It’s one way of explaining how environment impacts disease and behavior.”
Dr. Fowler’s group is learning more and more that environmental factors, especially in childhood, can dramatically influence phenotypes (behavior and disease). “We’re interested in enzymes that modify chromatin and cause changes in the brain. Drugs of abuse can have a profound effect on chromatin and gene expression. We’d like to be able to image these changes. So many problems start with the brain—behavior, addiction, violence, for example. If we can understand the molecular basis of that and develop treatment, it would make a huge difference.”
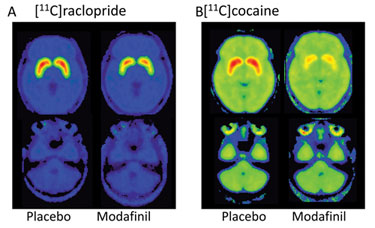
PET images of the brain from Brookhaven National Lab show that subjects given modafinil had lower levels of the radiotracer [11C]raclopride bound to dopamine receptors than subjects given a placebo.
Antibody Fragments
Although antibodies are now considered mainstream therapeutics, their potential for imaging agents as intact molecules is limited. They lack suitable pharmacokinetics and distribution within the body. This is the rationale behind the research of Anna Wu, Ph.D., professor of molecular and medical pharmacology at UCLA Medical School. “We want to capture the specificity of antibodies, but for imaging purposes.”
Her group has developed antibody fragments in three different formats to provide a variety of different pharmacokinetic and clearance patterns. The fragments are based on single-gene variable fragments, and re-formatting works the majority of the time. However, occasionally there is a loss of activity or affinity and this requires additional work to restore the desired binding.
Most applications being pursued are in cancer (solid tumors), using antibodies directed against cancer cell surface targets. “This approach allows us to control both the pharmacokinetics (Pk) and the primary organ of clearance. The Pk is important because we want to match that to the half-life of the selected isotope we’re using for imaging,” stated Dr. Wu.
The fragments include diabodies (55K molecular weight), minibodies (80K molecular weight), and single-chain Fv-FC (105 to 110K). The diabodies clear the body most quickly, followed by the larger fragments. This provides more control over where the fragments clear—diabodies clear via the kidneys and the larger fragments via the liver.
All fragments can be labeled with radionuclides for SPECT or PET. Dr. Wu’s group is focused more on PET because it is more sensitive and quantitative and is also a mainstay for oncology due to the availability and use of Fluorine-18 labeled FDG (fluorodeoxyglucose)—a glucose analog.
In the academic setting, Dr. Wu says they have developed about 6 to 12 fragments that have been used in preclinical mouse cancer models. In addition, Dr. Wu says that her group and others have published a few early studies evaluating engineered antibodies in the clinic. Commercially, she co-founded ImaginAb in 2007 to further develop these engineered fragments as clinical imaging agents.
ImaginAb has identified three disease areas with unmet clinical imaging needs and has in-licensed antibodies to develop imaging agents. “We’re partnering with pharma companies because these imaging agents can be useful along the drug development path—for example, helping to select patients for clinical studies and monitoring treatment response. Another one of our goals is to provide these companies with companion imaging biomarkers.”
Multidimensional Fluorescence
Researchers at Imperial College’s photonics department are developing multidimensional fluorescence imaging (MDFI) for cell biology, high-content analysis, and label-free tissue analysis. “The multidimensional part refers to the parameters you can get out of it, such as wavelength and polarization,” explained Sunil Kumar, Ph.D., research associate, photonics group.
Although fluorescence microscopy is a powerful functional imaging tool, in order to obtain quantitative images, one needs to apply spectral or temporal resolution. Since it’s best to avoid labeling live cells with extrinsic fluorophores, the MDFI approach of imaging autofluorescence (certain molecules like collagen and elastin are fluorescent) can distinguish different cell components and provide potential for label-free contrast. This approach is being applied to tissue imaging for research and clinical diagnosis.
Dr. Kumar’s group is also developing high-speed FLIM (fluorescence lifetime imaging), which can be used to image spatio-temporal organization of proteins and their organization. This uses an ultrafast shutter at different time delays after a laser pulse.
“You can build up intensities across your sample at different time delays and then put an exponential delay through them to get a lifetime image. Since you are doing this in a parallel fashion, you can put in a lot more light, acquiring images much faster,” explained Dr. Kumar.
This is an upgrade to time-coordinated single photon counting—considered the gold standard, which uses a single excitation via a pulsed laser to emit single photons. This takes a long time to build up an image. “You have to acquire hundreds to thousands depending on the lifetime decay to actually build up an image,” Dr. Kumar added.
Another technology currently under development is an automated high-speed optically sectioned FLIM multiwell plate reader applicable to fixed and live cells. “This looks for something in the sample that fluoresces and then automatically optimizes acquisition parameters. It will be used for drug discovery, especially if you have a particular biological system that expresses protein.”
A FLIM optical projection tomography system enables imaging of small samples, less than a centimeter in diameter. “It’s optically equivalent to a CT scan—you rotate the sample around its axis and collect data on it—and can do lifetime imaging at the same time, creating a 3-D lifetime map of the sample. We’re working toward getting images of whole mice.” Dr. Kumar said a potential application would be to record response to a drug or a particular activity.
Sidebar: Improving the Clinical Relevance of Early-Stage Research & Development
Given that one of the greatest drains on pharma return in investment is the exorbitant cost of clinical failure, it is imperative to ensure that in vitro and preclinical in vivo data is as predictive as possible of the clinical outcome. According to officials at Caliper Life Sciences, the company first recognized the critical nature of the “in vitro to in vivo bridge” in 2005 and subsequently developed it as the basis of its business model.
An abundance of recent technological developments, such as next-generation sequencing, genomic/proteomic biomarker discovery, companion diagnostics, and in vivo imaging with new modalities have strengthened this bridge, thereby facilitating more clinically relevant drug development, according to Caliper scientists.
Caliper has focused on the development of tools that specifically address key points across the in vitro to in vivo bridge, including genomics, proteomics, molecular profiling, and in vivo molecular imaging.
For example, Caliper’s IVIS® imaging system allows one to visualize the biological effect of a compound within a live animal, in real time, in a longitudinal manner, without the need to sacrifice the animal to obtain data.
“Not only does this reduce cost in terms of animal usage and labor (such as histology workload), but it provides real-time in vivo information that is more predictive of the compound’s activity in humans,” notes Mark Roskey, Ph.D., senior vp of biology R&D.
“With Caliper-engineered mouse and rat models for IVIS imaging across all the major disease indications, this platform is of broad utility to the pharmaceutical industry, and has been strategically used by many large companies to generate data for FDA regulatory approval of small molecule drugs, including Sutent® and Cubicin®” (developed by Pfizer and Cubist Pharmaceutials, respectively).
Going forward, Caliper intends to augment the in vitro to in vivo bridge by addressing molecular diagnostics, specifically by using Caliper tools to identify and measure biomarkers and to select key candidates for our new plastic chip assay format for evaluation as companion diagnostics, concomitant with the drug.
“By using a holistic approach and integrating data from multiple independent sources, we envisage creating a drug profile that will be more predictive of the drug candidate’s performance at the clinical stage and beyond,” adds Dr. Roskey.
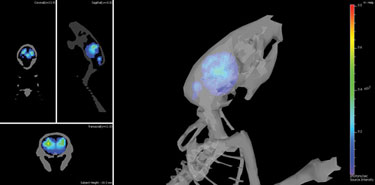
Brain inflammation caused by prion infection depicted using Caliper Life Sciences’ technology