May 1, 2009 (Vol. 29, No. 9)
Michael McGinley senior bioseparations product manager Phenomenex
Gregory Scott
Approaches for Better Characterization and Quantitation of Therapeutic Oligos
Synthetic oligonucleotides have been used as critical research reagents in the biotechnology industry since its beginning in the 1970s. Their increasing use first led to the formation of many core technology groups and eventually to an entire industry that revolved around providing custom synthetic oligonucleotides.
Methods for performing quality control analysis of these oligonucleotides have also evolved over the years, from using gels to current methods that include capillary electrophoresis, high-resolution ion exchange chromatography, and MALDI mass spectrometry. However, with the advent of oligonucleotide therapeutics as well as their widespread usage for in vitro diagnostics, there has been a growing need for methods that better characterize and quantitate both the oligonucleotide of interest as well as any synthesis contaminants.
HPLC using mass spectrometry detection (LC/MS) has been the method of choice for the analysis of protein and peptide therapeutics for quite some time, but was not widely used for characterization of oligonucleotides due to incompatibilities between the ion pairing mobile phases used for reversed-phase (RP) separations of oligonucleotides and mass spectrometry.
Ion-pairing reagents are mobile-phase additives used to increase the retention and resolution of polar compounds being separated by reversed-phase HPLC. High molarity amounts (100 mM triethylamine acetate [TEAA]) are typically used for RP separation of oligonucleotides, however the high level of TEAA leads to ion suppression that makes MS detection of oligonucleotides difficult at therapeutic and reagent concentrations.
New LC/MS methods have been introduced in the last few years that use a combination of triethylamine (TEA) at low levels and hexafluoroisopropanol (HFIP) as a mobile-phase buffer. For RP separations this mixture has enabled reasonable sensitivity by MS.
An example of a typical oligonucleotide LC/MS run is shown in Figure 1; a 21 mer DNA oligonucleotide is run on an Agilent 1100 HPLC using a Clarity Oligo-RP HPLC column from Phenomenex. MS data is collected on an ABI 3500 triple quad MS system (Applied Biosystems). The MS total ion chromatogram (TIC) in Figure 1a shows the main peak for the 21 mer oligo; the raw MS spectra of the major peak is displayed in Figure 1b. Note that the spectra contain ions corresponding to the full-length oligonucleotide in the -4 through -9 charge states.
Key to determining any structural changes or quantitating any minor components in an oligonucleotide sample by LC/MS is having deconvolution software that can calculate the contributions of these multiple ions into a parent mass. Figure 1c shows the expected reconstructed mass for the full-length oligonucleotide generated by ABI Analyst software (Applied Biosystems); minor components can also be seen in the reconstructed mass.
From this example it is readily apparent why deconvolution software is needed to interpret oligonucleotide MS data; the utility of LC/MS analysis for characterizing oligonucleotides is easily seen. Although not shown here, additional information regarding the nucleotide sequence and location of any oligonucleotide modifications could be obtained by performing MS/MS analysis of any oligonucleotide signal and analyzing the resultant daughter ion spectra.
While computational solutions for dealing with complex MS data are important for characterizing oligonucleotides, a more critical part of performing LC/MS analysis is maximizing sensitivity and resolution of the minor components in an oligonucleotide sample. Whether it is for impurity analysis or pharmacokinetic metabolite studies, chromatographically separating minor components is a requirement because many low-level impurities are isobaric and need to be somewhat resolved to aid in characterization and quantitation. MS sensitivity is also important because many of these minor components are already at the limit of detection using current LC/MS methodologies.
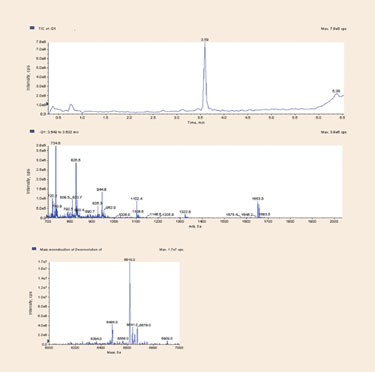
Figure 1. LC/MS analysis of a 21 mer DNA oligonucleotide using a Clarity Oligo-RP HPLC column
Improving Sensitivity
Recently efforts were undertaken to improve sensitivity of oligonucleotide LC/MS analysis while maintaining good resolution between different oligonucleotides. An HPLC column specifically designed for oligonucleotide separations, Clarity Oligo-RP, was used because this type of media will most likely show chromatographic changes during method optimization studies.
As MS sensitivity to oligonucleotides is believed to be most influenced by the MS suppression effects of ion-pairing reagents, different levels of ion-pairing reagents were evaluated for their effects on an oligonucleotide separation. A poly-dT 12–18 standard was used because the mixture is widely accepted in the industry as a good selectivity measure of a column’s performance. An overlay of the poly dT standard using different mobile phase conditions is shown in Figure 2.
Contrary to popular belief, MS sensitivity actually decreased with reduced ion-pairing reagent present in the mobile phase. Resolution also was seen to decrease with decreased ion-pairing reagent as retention of all analytes was greatly reduced. This reduction in analyte retention is actually the key factor in understanding MS sensitivity for oligonucleotides; the loss of ion pairing influenced retention results in oligonucleotides eluting in lower percentages of organic mobile phase.
It is well known that electrospray LC/MS efficiency is influenced by the percentage of organic present; increased retention of oligonucleotides caused by ion-pairing reagent makes up for any losses due to ion suppression, up to a point. The separation using 15 mM TEA/ 400 mM HFIP (a common concentration cited in the literature) shows a significant loss in MS signal due to ion-suppression effects. Thus it appears that there is an optimal balance between retention and ion suppression in maximizing LC/MS sensitivity for oligonucleotides.
For the Clarity Oligo-RP column that level appears to be around 2–6 mM TEA and 100–300 mM HFIP (these amounts are likely unique for each reversed-phase column).
In an attempt to further optimize both resolution and MS sensitivity, ratios between TEA and HFIP were investigated by fixing the amount of HFIP used (200 mm) for oligonucleotide separations and varying the levels of TEA used in the mixture. By varying the TEA and keeping HFIP constant, the pH of the mobile phase will change, giving some indication of the influence that pH may play on this separation.
Figure 3 is an overlay of the poly dT standard run at different HFIP/TEA ratios. Unlike the previous figure where dramatic changes in retention, resolution, and sensitivity are observed, varying the buffer ratios has a much less pronounced effect on sensitivity, though resolution did differ significantly between analyses.
Again there appears to be an optimal ratio between extremes, in this example using the Clarity Oligo-RP column the best condition evaluated was a mobile phase of 8 mM TEA with 200 mM HFIP (pH 8.0) in the aqueous mobile phase. These conditions provided both the best resolution and retention for the oligonucleotide mixture (which again will have an influence on sensitivity).
While these experiments only show an optimized separation for one oligonucleotide mixture (poly dT oligonucleotides), a common theme in both mobile-phase experiments is that there is an optimal balance of mobile-phase buffers that increases resolution and sensitivity for LC/MS applications. Such a methodology can be applied to other types of oligonucleotides (DNA, RNA, and phosphorothioates) to optimize a particular application. Of course, a key to success for any of these applications is proper instrumentation, column chemistry, and computational tools to generate, analyze, and interpret the oligonucleotide LC/MS data.
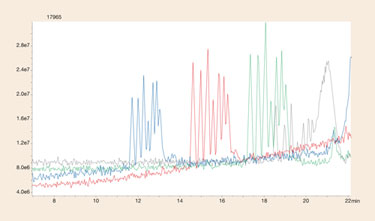
Figure 2. LC/MS separation of a 12–18 poly dT oligonucleotide at different ion-pairing concentrations using Clarity Oligo-RP
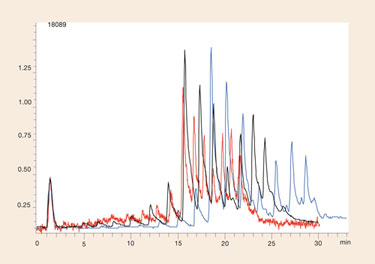
Figure 3. Influence of ratios between HFIP and TEA on LC/MS sensitivity and resolution using Clarity Oligo-RP
Michael McGinley ([email protected]) is a product manager, and Gregory Scott is a research scientist at Phenomenex. Web: www.phenomenex.com.







