November 15, 2011 (Vol. 31, No. 20)
Angelo DePalma Ph.D. Writer GEN
Diverse Methodologies and Fresh Insights Turn a Good Technique into an Even Better One
Antibody engineering holds the potential for creating antibodies with multiple specificities, greater affinity for their targets, and fewer side effects. Among these approaches, more-or-less traditional protein engineering remains a topic of intense interest.
Zymeworks whose trademarked tagline is “Building Better Biologics™,” describes its technology as “structure-guided design.” Those who remember classic medicinal chemistry will recognize the synonymous “structure-activity relationship.”
That proteins could be designed in this manner makes sense, but the idea went into hibernation after the initial hype because the tools for correlating structure to activity were primitive. “Our understanding of protein dynamics was not very advanced, not like today’s,” says Ali Tehrani, Ph.D., Zymeworks’ president and CEO.
Techniques like NMR, crystallography, and protein modeling were simply not up to the task. Now that these analytics have caught up, investigators can exploit molecular biology to its fullest.
A typical protein-engineering exercise might begin with a wild-type antibody and identification of the critical regions and amino acids responsible for binding. These regions are analogous to “functional groups” in small molecule drugs.
“We then ask which amino acids can be replaced such that desirable properties are improved, while retaining the molecule’s other critical properties,” Dr. Tehrani explains. This resembles site-directed mutagenesis, but instead of a single amino acid, Zymeworks looks for groups of building blocks that need not necessarily be contiguous. The molecular modeling engine behind this work is ZymeCAD™, which performs in silico mutagenesis to create candidate antibodies.
Zymeworks employs two molecular scaffolds: Azymetric™, an IgG1-based platform consisting of two heavy chains assembled onto one molecule, and AlbuCORE™, a multivalent antibody alternative. Being antibody-like, Azymetric can recruit T cells and bind to two antigens at the same or different sites. Azymetric is appropriate for treating cancers due to its bispecificity and effector activity.
“Rather than applying pressure to one point, you apply it to two points and create a much stronger signal for tumor destruction,” Dr. Tehrani explains.
AlbuCORE is more suited to treating cardiovascular, inflammatory, or auto-immune diseases because it does not recruit immune system cells.
In late September, Zymeworks revealed a new round of financing worth $8.1 million. Less than a month earlier, MSD/Merck signed a nonexclusive agreement with Zymeworks for developing bispecific antibodies.
Immunogenicity is known to limit dosing of certain mAbs, so eliminating it is of great interest. Antitope provides antibody humanization and protein-engineering services that focus, through in vitro and in silico tools, on screening out potential immunogenicity during preclinical development. The company also maintains a pipeline of its own molecules.
“If we start with an early-stage molecule, we can rationally design it to avoid T-cell epitopes, which are key drivers of immunogenicity,” explains chief scientist Matthew Baker, Ph.D.
Antitope concentrates on helper T-cell epitopes, not post-translational modifications. Accumulating evidence supports this approach for therapeutic proteins, including antibodies, and for drug candidates derived from plants and bacteria. The latter are more challenging than conventional proteins from an immunogenicity perspective, largely because the human body more readily recognizes these molecules as nonself.
“Depending on the bacteria, patients may have already been primed against these proteins through previous exposure,” Dr. Baker says.
Antitope’s “de-immunization” technologies amount to mapping T-cell epitopes in the sequence and removing them by point mutation, which disrupts binding to MHC. This approach, applied predominantly to antibody variable region sequences, combines elements of standard humanization with de-immunization and generates sequences that appear human in origin.
The challenge with conventional immunization technologies was incorporation of single-point mutations that disrupt a protein’s affinity for MHC without affecting stability, target binding, and efficacy. “This requires screening large numbers of sequences,” says Dr. Baker.
Tandem humanization/de-immunization combines sequence data from a large database of human variable region sequences with in silico structural analysis. Thus, Antitope can select sequence segments from unrelated, fully human antibodies and stitch them together to generate composite molecules possessing the specificity on par with nonhuman reference antibodies.
Antitope’s internal pipeline consists of seven early-stage molecules, with two that show “good efficacy” in animal models. “And obviously, we’ve addressed any immunogenicity issues as well,” Dr. Baker adds. Antitope plans to out-license its advanced drug candidates after demonstrating proof of concept.
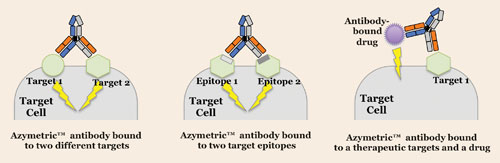
Azymetric antibodies are engineered to have multiple disease target binding domains to induce better cell signaling and/or cell death. Azymetric antibodies can simultaneously: bind to two different synergistic targets (left), bind to two different epitopes on the same cellular target (middle), and bind to a cellular target while delivering a bound drug or toxin (right), according to Zymeworks.
Animal Systems
Animals expressing genetically engineered, fully human antibodies hold tremendous promise, but access to these systems has not been straightforward. Abgenix made its name on the Xenomouse™, and Medarex with its KM Mouse™.
But Abgenix was acquired by Amgen, and Medarex by Bristol-Myers Squibb. That leaves only Regeneron’s Velocimmune mouse as a readily available—but costly—platform for generating humanized antibodies. For example, Regeneron’s two licensing deals, with Astellas and AstraZeneca, could net more than $350 million.
“It became extremely difficult and expensive to access this technology. That’s why I founded OMT [Open Monoclonal Technology; www.openmonoclonaltechnology.com],” says CEO Roland Buelow, Ph.D. His previous venture, Therapeutic Human Polyclones, which used genetically engineered rabbits to express a human antibody repertoire was acquired by Roche in 2007.
According to Dr. Buelow, OMT’s rat platform expresses fully humanized antibody as easily as rats make rat antibodies.
Rats are an ideal expression model because they exhibit very little, if any, of the strong selection pressure. By contrast, rabbits, mice, and cows endowed with human IgG loci produce very little fully humanized antibody—around 1%.
Expressing fully humanized antibodies in animals is a two-step process: first create a knockout animal by inactivating their natural antibody genes, then introduce recombinant immunoglobulin loci that encode for antibodies with fully human idiotypes. This had never been done before in rats. “It was technically difficult, but no new technology was required,” Dr. Buelow says.
At the time he founded OMT in 2008, no applicable nuclear transfer cloning protocol existed for rats. Dr. Buelow instead used zinc finger nucleases—DNA-binding proteins that generate mutations in precise locations in the genomes of higher animals.
Single-Domain Antibodies
Single-domain antibodies are small proteins derived from the heavy-chain VHH regions of conventional antibodies. Their discovery, by researchers at the Free University of Brussels, in the early 1990s, was based on the observation that antibodies in camels and llamas lack light chains.
Researchers then discovered that VHH regions alone bound to antigens as strongly as the intact antibody and were extremely stable. Therapeutic antibodies have molecular weights of about 150 kDa, whereas single-domain molecules weigh in at under 15 kDa.
Single-domain antibodies overcome problems with mAbs related to their large size, chemical lability, expensive manufacturing, and inability to penetrate into cells.
In 2001 the Free University of Brussels spun off Ablynx, which owns the exclusive commercialization rights for the technology for human therapeutics and diagnostics. Ablynx, which calls its molecules Nanobodies, now boasts a robust pipeline of over 25 programs with three drugs in late Phase II clinical development, four in Phase I, and the remainder in preclinical development. About 50% of these programs are partnered with Pfizer, Merck-Serono, Novartis, and Boehringer Ingelheim.
In May 2011, data released from a Pfizer-led Phase II study of a Nanobody against TNF-alpha demonstrated proof of concept in patients with rheumatoid arthritis, Ablynx reports. “This validates the technology,” notes Ablynx senior research fellow Hilde Revets, Ph.D.
So far Nanobodies have lived up to their promise of manufacturability, solubility, and versatility. Since they comprise only the VHH domain harboring the full antigen-binding capacity of the original heavy chain antibody, Nanobodies may be produced in prokaryotic hosts. Glycosylation has not been an issue thus far, but Dr. Revets notes that undesirable “hot spots” for undesirable post-translational modifications may be deleted out of Nanobodies via sequence optimization.
The strict monomeric behavior and small gene size make Nanobodies ideal building blocks for multivalent (two or more identical Nanobodies on one molecule) or multispecific (two or more different modes of activity) therapeutics. This level of tailorability combined with solubility and ease of manufacturing properly folded protein is difficult to achieve with conventional antibodies, which are already quite large, more difficult to format, and sometimes exhibit solubility issues.
The group at the University of Brussels remains active in this research, as are other academic labs. It is unclear, however, whether Ablynx controls the method of production, the chemical entities, or both.
For example, Ablynx develops nanobodies by vaccinating llamas, collecting blood, isolating mRNA, converting it to DNA, and expressing the protein in yeast. Alpha Universe skips the vaccination step. “Everything is done in vitro, which is less expensive,” says Alexey Zdanovsky, Ph.D., president. Not surprisingly, he would not disclose his method for creating the truncated antibodies, only that it involved “molecular mutagenesis selection.”
Bispecific antibodies provide the potential of two modes of action in one molecule. In early October, X-Body entered into a partnership with Tanabe Research Labs to identify therapeutic target epitopes and develop mono- or bispecific antibodies for autoimmune disorders. X-Body says it will screen its diverse human libraries against target cells to identify specific epitopes and antibody therapeutic candidates.
X-Body screens its human antibody library using its Protein Chain Reaction™, which screens against cell surface targets in their native states on live cells or purified target proteins. According to the company, this “deep sequencing hit analysis” identifies and characterizes rare antibodies with specific therapeutic properties.
“Our nucleic acid display is the most compact approach compared with phage, yeast, or mammalian cell display,” says Tod Woolf, Ph.D., vp of technology development, “and it’s extremely diverse.” The other advantage is that these are fully human antibodies displayed in a mammalian system. “When you use phage display, proteins don’t fold the same way or have the same codon bias.”
X-Body’s library was generated from human donors’ antibodies. Variable chains were amplified using PCR, which captured the full diversity. “Our library is unusual in that it is not engineered,” Dr. Woolf says.
In 2001, a group at Genentech that included senior scientist Mark Dennis, Ph.D., reported increased uptake and activity in the brain of a bispecific mAb. One arm of the antibody binds to the transferrin receptor (TfR), which is upregulated on the surfaces of brain endothelial cells; the other arm inhibits b-secretase 1 (BACE1), an enzyme that transforms amyloid precursor protein into b-amyloid. Amyloid plaques are well-known markers for Alzheimer disease.
mAbs have the potential for treating brain diseases, but the blood-brain barrier excludes them: Just 0.1% of the antibody present in blood enters the brain.
The TfR receptor presents an interesting entry point through the blood-brain barrier. At one time researchers hoped that drugs binding to TfR would be internalized if they adhered to the receptor long enough.
“This receptor-mediated transcytosis strategy was proposed 20 years ago,” Dr. Dennis says. But later studies showed that strong binding tends to keep the antibody trapped inside blood vessels. “So the simple-minded idea is to lower affinity to TfR so the antibody can be released into the brain.”
The Genentech group attenuated TfR affinity by inducing alanine mutations in the complementarity-determining regions of the mAb. In a therapeutic setting, the drug concentration is sufficient to overcome the weaker binding affinity and drive antibody uptake by TfR, which does the rest.
Compared with a control antibody specific for b-secretase 1, the bispecific anti-TfR/anti-BACE1 antibody accumulated in the mouse brain and reportedly led to a significantly greater reduction in b-amyloid in the brains of test mice.



