May 15, 2011 (Vol. 31, No. 10)
Timothy A. Moeller
Technique Addresses Inhibition and Induction to Improve Screening for Drug Safety
Advancement of techniques to achieve efficient and relevant outcomes occurs when technologies merge at critical moments. In the pharmaceutical industry, techniques such as in silico drug design and microarray analysis illustrate the power of combined technologies to transform the manner and expectations of how pharmaceutical research is performed. The refinements allow for not only greater generation of data but also deeper understanding of that which is observed.
Drug safety advances have also been notable. The combination of high-throughput screening (HTS) technology, development of substrate probes, and quality of relevant cellular systems has led to the meaningful development of techniques for the assessment of drug-drug interactions (DDI). Traditionally, DDI occur when one drug affects a second drug’s efficacy or toxicity due to perturbing the metabolic capacity of a patient by the inhibition and induction of cytochrome P450 (CYP) enzymes.
Inhibition
Inhibition of CYP enzymes diminishes the metabolic activity of an enzyme, resulting in accumulation of the drug that reduces therapeutic efficacy or increases toxicity. For example, bergamottin, a component of grapefruit juice, inhibits CYP3A4, the enzyme responsible for the metabolism of more drugs than any other enzyme. Clinically significant DDI have been associated with consumption of grapefruit juice and numerous drugs that are metabolized by CYP3A4 such as midazolam and atrovastin.
To test for this phenomenon in vitro, microsomes, the subcellular fraction containing CYP enzymes, have been used as the gold standard. However, results from microsomal inhibition may not correlate to in vivo responses due to other cellular events. The use of hepatocytes in lieu of microsomes leads to better in vivo correlation of clinically relevant inhibition when intracellular concentrations of a drug are affected by drug transporters or other events.
However, the use of hepatocytes to augment or potentially replace microsomes as the primary system for inhibition studies requires these cells to emulate key microsomal attributes while increasing the quality of data due to whole cell architecture.
To address these needs, cryopreserved pooled human hepatocytes have become readily available to the research community from companies such as Celsis, providing an average of responses from multiple donors to mitigate idiosyncratic responses and offering consistency among lots to minimize revalidation of reagents.
This mimics the power of averaging CYP enzymatic activities found in human liver microsomes and, in addition, includes the full complement of Phase I and Phase II drug metabolism, uptake transporter activities, and cell membrane barrier.
The utility of human hepatocytes to determine inhibition has been demonstrated in recent studies by comparing the inhibition of four clinical drugs with known transporter-enzyme interplay. In one study, the authors concluded that determination of inhibition and enzymatic activities is not intuitive between microsomal and hepatocytic systems, and highlighted potential shortcomings when using microsomes to predict clinical responses.
Promega, BioTek Instruments, and Celsis collaborated on research that was presented in a poster at “LabAuto” earlier this year. The poster described the use of pooled human hepatocytes in HTS format to measure inhibition of CYP enzymes. The authors developed a high-quality 384-well system for dispensing and incubating pooled human hepatocytes, as well as measuring hepatocytic responses using luminogenic specific substrates.
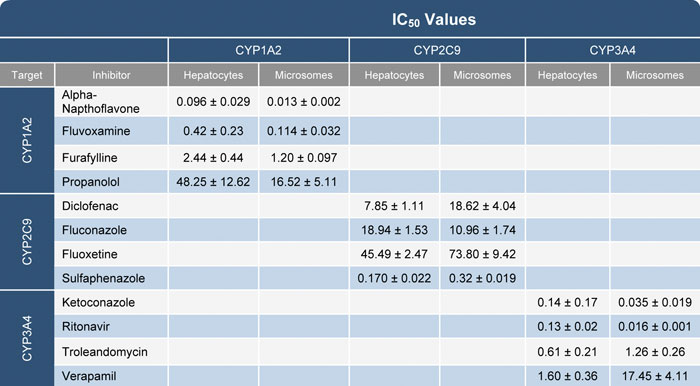
The results of the inhibition using pooled human hepatocytes were compared to human liver microsomes under the same conditions. The derived inhibition constant Ki was similar for most of the test compounds except for a few with known transporter interactions like verapamil (Table).
Pharmaceutical researchers now have a new technique to further study inhibition in a more relevant manner, while retaining the work flow expected with microsomal inhibition assays.
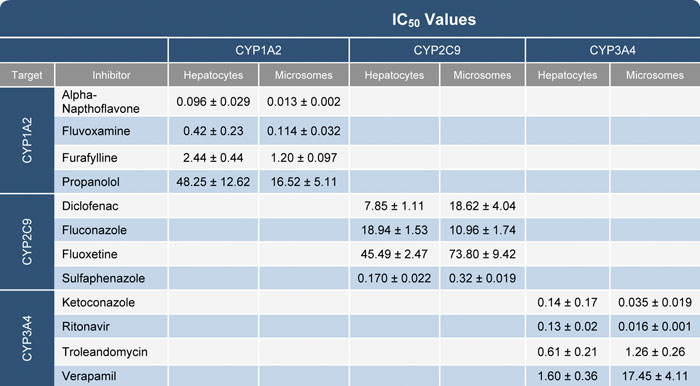
Table. IC50 values generated from cryopreserved pooled human hepatocytes and human liver microsomes using P450-Glo substrates for CYP1A2, -2C9, and -3A4.
Induction
Induction of CYP enzymes increases the metabolism of a drug, resulting in a reduction of efficacy of the intended therapy. For example, certain antibiotics like rifampicin can increase the metabolism of birth control drugs, rendering them ineffective.
As with inhibition DDI, induction potential must be assessed prior to exposure in patients. Human hepatocytes are the gold standard for determining induction potential. However, the commonly used 24-well format limits the number of wells per plate and the cost per well. Therefore, a migration to 96-well and 384-well induction models would offer a higher capacity to generate the required data with the same resources.
Though hepatocytes in 96-well format have been shown to be an appropriate model for induction, the 384-well format has not been reported in the literature. A second poster, presented by the group at “ISSX”, described validation of a 384-well format. The 96- and 384-well formats were compared to ensure that miniaturization of the system did not impact the function of the hepatocytes or the derived values. To increase the quality of the data, a triplex system was developed to measure CYP1A and -3A4 activities along with viability from a single well.
In doing so, the efficient use of hepatocytes and dosing solutions reduced potential variability from inter-well and inter-plate cell culturing and multiple drug-dispensing steps. That is, from a single dispensed dose, functional data was obtained for two induction endpoints and a viability endpoint. The CYP1A family was measured by the fluorescent substrate ethoxyresorufin, CYP3A4 by the luminogenic substrate P450 Glo™ CYP3A4 IPA (Promega), and viability by the luminogenic assay CellTiter-Glo®.
The authors presented a 384-well system with robustness and consistency that meet HTS criteria while retaining the induction mechanism of human hepatocytes. In addition, the viability assessment allowed for normalization of the data as well as the potential to explain differences in the EC50 curves.
For example, rifampicin showed lower induction at the high end of the curve while the viability remained constant, which may be interpreted as a suppression of the induction process or the inhibition of CYP3A4 enzyme (Figure, A).
In contrast, lansoprazole showed a lower induction at the higher doses along with lower viability, allowing for the interpretation that toxicity of lansoprazole was responsible for the lower induction (Figure, B).
The combination of an HTS platform and triplex methodology with human hepatocytes creates a powerful new technique for pharmaceutical scientists to assess induction potential. In addition, triplex methodology provides for efficient use of resources such as hepatocytes and dosing solutions, for reducing variation in responses compared to combining separate data points, and for normalization of data by viability parameter. The latter provides a critical value to explain a deviation from a sigmoidal response curve due to cellular toxicity or other cellular events.
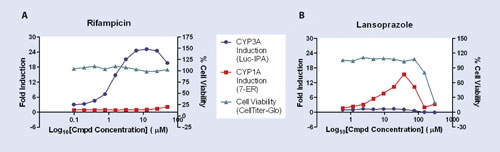
Figure. Induction responses for CYP1A2 and -3A4 as measured by metabolism of ethoxyresorufin and P450-Glo™ 3A4 IPA substrates, respectively, after treatment with rifampicin (A) and lansoprazole (B). The fold induction was derived by dividing the metabolic activity of the treated hepatocytes by the metabolic activity of the untreated hepatocytes. Viability was measured by ATP content using CellTiter-Glo.
Integration
The responsibilities of pharmaceutical scientists have increased to meet a variety of challenges. The pharmaceutical industry is being pressured by regulatory agencies to provide extensive preclinical data to assess drug safety issues, such as DDI, before embarking on clinical development of new drugs. In addition, pharmaceutical scientists have internal pressure to screen more compounds with limited resources while producing clinically relevant and actionable information.
New technologies and integrated platforms must be developed to meet these evolving needs. Two such platforms, HTS induction and inhibition systems using human hepatocytes, offer the potential to meet the challenges of today’s drug discovery programs by merging formerly separate technologies and reagents in order to provide new and potent techniques.
Timothy Moeller ([email protected]) is scientific advisor at Celsis.