May 15, 2009 (Vol. 29, No. 10)
Chris Walsh
Advanced Resolution Vision System Is Designed to Improve Sample Quality
Significant sums of money have been invested in developing automated systems aimed at boosting the throughput, efficiency, and cost effectiveness of sample screening. Investments made in this area are wasted, however, if the quality of the sample in a well plate is not accurate. Empty wells, or wells with the wrong concentration, result in wasted reagents, repeat screens, increased costs, and potentially false negatives.
The integrity of samples within an automated storage system has a significant impact on the quality of the delivered plate wells and the resulting screening process. Knowing the volume of sample within the source tubes and whether any sample has precipitated out of solution, resulting in a different concentration, are key requirements for compound management and high-throughput screening groups.
Current automated techniques for checking the volume of a sample in a tube are both time consuming and inaccurate, while manual methods are simply impractical when dealing with HTS or large compound libraries due to the numbers of samples that must be audited. Manual visual checks at a pharmaceutical company, which handles millions of sample tubes, would be difficult and expensive to perform on a regular basis.
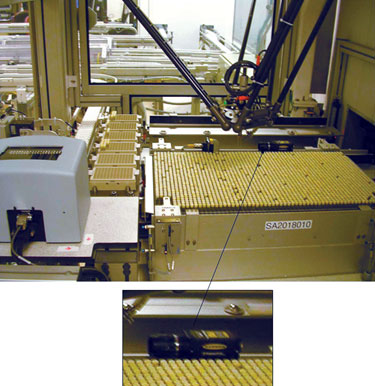
A RTS automated storage system with high-speed tube picker. The insert shows a camera.
Finding Volume in a Tube
Traditionally, the sample volume in a tube is found by knowing the tare weight of the tube, which is then subtracted from the gross weight of the tube, i.e., tube plus solubilized sample contents. This process is not only time consuming to perform manually, but also requires software that links the resulting data back to the sample inventory.
Technological advances have resulted in the introduction of instruments designed to assist with this method of volume determination. Typically, such instruments process a rack of tubes by selecting a single tube and scanning its bar code before then weighing it. The data is stored on file and linked back to the sample inventory. While these devices can also be used to pretare tubes, there are a number of limitations. Caps or septa, used to seal the tubes, can sometimes vary in weight by as much as 50 mg; this means that even with automated solutions, the weighing of a sample tube may not ensure that the volume information is accurate.
Another automated approach intended to address this issue utilizes acoustic sensor technology. Sensors, placed above the open necks of the tubes, allow the calculation of the distance to the upper surface of the liquid (and hence the volume in the tube) by measuring the time for the acoustic signal to reflect back from the meniscus.
While acoustic methods allow relatively high-speed volume auditing, there are again limitations when using this technique. For example, the signal from an acoustic sensor can be affected by several environmental factors that may lead to false information being returned. In addition, a calibration data set must be generated from previously measured sample volumes. Finally, and perhaps most significantly, this method only works with un-capped tubes, which may have significant implications for sample quality. Determining precipitates is also not possible using this technique.
Perhaps the simplest approach to tracking sample volume is to just maintain a calculated value in an inventory database, and decrement that value in line with the sample volume removed from the tubes during liquid-handling operations. This approach, which has been adopted by many groups, relies on the liquid handling being performed exactly as intended. However, blocked tips, or double aspirate/dispense cycles are not unknown, both of which will result in the tracked volume soon becoming inaccurate. The benefit of a closed-loop measurement process is thus clear.
Sample Concentration
If a sample is at the wrong concentration, this also will have a significant impact on the downstream screening process. Sample precipitation can result in the concentration of the sample dispensed into the plate well being quite different from what was intended; a low concentration may ultimately result in the return of false negatives.
Sample precipitation can be a significant problem in sample storage and management; there are many varying factors that can lead to precipitation, including how long a sample has been stored, the storage temperature and conditions, and the initial sample concentration. Moisture uptake, in particular, is problematic, causing samples to come out of solution during repeated freeze-thaw cycles.
Traditionally, manual visual inspections have been the only way of checking whether a sample has precipitated out of solution as no automated process has been available.
Vision System
Emerging technology has allowed the application of high-resolution vision systems to address both volume measurement and precipitate detection. An example of such a solution, available from RTS Life Science, uses a camera and light source mounted within an automated storage system picking station (Figure). The camera captures images of the tubes as they are selected and transferred to/from the storage tray. The image of the sample tube is analyzed, and the height to the top of the liquid in the tube is calculated. This height measurement is then converted to volume depending on the physical characteristics of the tubes in question. Each image may be stored for later recall if required.
Volume measurement using this approach can offer substantial benefits by overcoming many of the limitations inherent with the other techniques already described. When measuring volume using vision, the need to pretare tubes is eliminated; inaccuracies arising due to weight variation, whether tube or cap, are no longer a concern. In addition, this method means there is no longer a need to remove the cap, ensuring the quality of the audited sample is not compromised. As with most vision technology, operation is extremely quick, meaning that high-speed auditing is now a reality.
Finally, and perhaps of greatest benefit, by suitable processing of the captured image, it is now possible to identify the formation of precipitate within the sample.
By integrating this technology into an automated storage system, it is also possible to allow compound managers to determine when a tube should be audited. Specific samples can be audited on-demand or a trigger-threshold can be set to define when an audit should be performed; for example, when a sample has been pipetted from a specified number of times. This gives greater control to the compound manager over the auditing process and means that frequent checks can be scheduled without the need for any manual intervention.
Cost savings are brought about through the increased accuracy of the data returned by the vision system, thus reducing the risk of empty wells, or wells with the wrong concentration, which would otherwise result in wasted reagents and repeat screenings.
As a compound manager it is important to know as much about the quality of the sample library as possible. Where traditional techniques have proved to be costly and time consuming, emerging technologies including the RTS Life Science vision system are now providing a thorough and accurate method of sample volume measurement. By applying new technology and techniques to auditing processes, compound managers can be more confident about information relating to volume levels in their samples, as well as gain access to previously unavailable data such as sample precipitation.
High-resolution vision overcomes some of the key limitations of other techniques; it also increases speed and efficiency by eliminating time-consuming and error-prone processes and also ensures sample integrity is maintained throughout. Automating this technology means that companies with a high throughput can now run frequent sample checks where previously this was just not practical.
Chris Walsh ([email protected]) is sample-management product manager at RTS Life Science. Web: www.rtslifescience.com.







