March 15, 2015 (Vol. 35, No. 6)
Angelo DePalma Ph.D. Writer GEN
To Generate Therapeutic Glycoproteins of Consistent Quality, Bioprocessors Must Control for an Abundance of Variables
Glycosylation of therapeutic proteins is nearly always mentioned within the context of quality and consistency. Critical quality attributes (CQAs) are affected by multiple factors, including clone selection, culture and purification conditions, formulation, and storage conditions.
For Elizabeth Dodson, Ph.D., an R&D manager in the advanced bioprocessing unit of BD Biosciences, the key attribute is primary structure followed by post-translational modifications, particularly but not limited to glycosylation. Focusing on the effects of media and feed supplementation on quality, Dr. Dodson’s group has developed an automated, high-throughput technique.
The technique starts with the purification of monoclonal antibodies from cell culture supernatants. This procedure is followed by a semi-automated protocol for isolating, purifying, and characterizing N-glycans with results comparable to manual techniques but with at least 10 times higher throughput. Dr. Dodson’s approach employs high-throughput screening at the earliest stages of clone selection, followed by more detailed examination of candidate clones.
BD Biosciences’ scientists begin by growing clonal cell cultures in shaking deep well plates, allowing more clones at early development stages. They then move to Agilent’s AssayMAP Bravo robotics platform, which processes 96 samples at a time and purifies IgGs from the spent media in about four hours. “Having nearly one hundred samples to examine this early is the tremendous benefit for large-scale screening at the front end,” Dr. Dodson says.
Samples then undergo standard glycan analysis, followed by UPLC-FLD whose operations, including peak selection, are fully automated. Dr. Dodson estimates that using manual assays of equivalent sophistication, developers may process five samples per week. Her approach blasts through 96 samples in four hours.
Automating analysis should be a boon to biosimilar developers attempting to match the glycosylation profile of their molecule with the originator’s. Because the glycans coming off the antibodies are very well-characterized, it is possible to discern even structural isomers.
Dr. Dodson believes that clonal selection is the most critical factor in protein quality. “If you select an early clone for titer, you might reach the end of a full-optimization project to discover that the clone cannot provide the quality or glycosylation pattern you’re looking for,” she advises. “By screening more clones earlier, customers have a better chance to find cells that provide the desired protein quality.”
Once this cell line is established, developers can proceed with other aspects of process or media optimization, with the knowledge that the biochemical pathways responsible for CQAs are firmly in place. “If the cell is unable to do that from the beginning,” Dr. Dodson notes, “you can’t fix it by adding something in the middle or purifying it differently.”
BD Biosciences’ approach cuts against the grain for developers for whom automation is mysterious, or who lack the appropriate analytical expertise. Reliable glycan analysis is a relatively new capability limited to companies with broad analytical acumen or access to partnerships. “If you don’t have a way to purify a large number of clones quickly, and characterize them in a semi-automated fashion, you drive yourself nuts by doing them three at a time,” Dr. Dodson adds.
Homogeneity
Glycosylation’s status as a CQA is based partly on its effect on pharmacokinetics. Prescribers and patients benefit from knowing how long a drug circulates in the bloodstream, which is why the greater the homogeneity of glycosylation the better.
Conventionally, developers improved heterogeneous glycosylation through genetic modification of cell lines and/or process optimization, but these approaches produce variable results. For these reasons a group at Roche Diagnostics has turned to in vitro glycoengineering.
“For some therapeutic proteins, certain glycoforms improve pharmacokinetics or biologic function,” says Roland Dorn, international product manager at Roche custom biotech. Sialylation, for example, improves pK. In vitro glycoengineering can enrich the preferred glycoform in the final drug substance, while reducing characterization efforts aimed at demonstrating comparability between scales or batches.
Factors that tend to improve expression or yield can negatively affect homogeneity achieved by media optimization or cell-line engineering (e.g., through over-expression of glycosyltransferases). “These approaches can be time- and cost-consuming, whereas in vitro glycoengineering can be used independent of the production process for new or existing products,” Dorn tells GEN.
In vitro glycoengineering involves post-process enzymatic modification of the glycosylation pattern. The target protein is incubated with a highly specific beta-1,4-galactosyltransferase or alpha-2,6-sialyltransferase and the corresponding activated sugar (UDP-Gal, CMP-NANA). This leads to the formation of galactosylated or sialylated glycoforms, which are therefore substantially enriched. “The use of well-characterized enzymes in these reactions leads to predictable outcomes and reduces complexity,” Dorn adds.
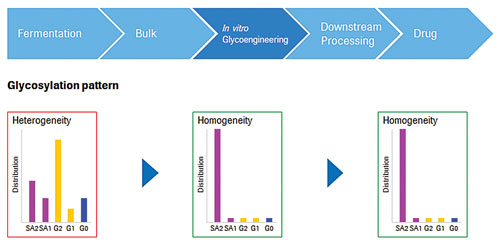
In vitro glycosylation technology from Roche Custom Biotech can help bioprocessors engineer the glycosylation pattern of therapeutic proteins such as recombinant monoclonal antibodies. Directed modification by means of highly active glycosyltransferases, activated sugars, glycosidases, and other tools can increase glycosylation homogeneity.
Black Box Approach
Understanding the relationship between cellular responses and process variables facilitates development of control processing schemes for fed-batch and perfusion bioreactors. To achieve this, a group at Bend Research, part of Capsugel Dosage Form Solutions, directed by Jeff Breit, Ph.D., employs time course datasets that quantify kinetic responses of cells to changes in process parameters through an experimental methodology that “excites” process dynamics.
One result of this effort has been identification of process inputs that positively affect protein quality as determined by glycosylation control in a perfusion bioreactor. Dr. Breit believes this methodology will translate to fed-batch systems as well, thereby greatly broadening the approach’s applicability.
“We treat the bioreactor like an engineered process, and we’re perturbing one variable at a time to understand how aspects of the systems affect variables like sugar residues,” Dr. Breit says.
Here the principal input is galactose concentration, and the output galactosylation. However, the Bend Research approach could operate through perturbation of any other variable—media components, cell metabolite levels, process features—that might affect galactosylation or any other quality attribute such as deamination, oxidation, protein fragments, or lactate/ammonia production.
Dr. Breit describes the Capsugel/Bend Research work as an “input/output” approach that should work with any cell-line or glycosylation optimization project. What differentiates this method from other development platforms is the ability to sample the bioreactor rapidly, using Bend Research’s aseptic sampling technology (MAST™), in near-real-time. Data is then channeled through a software suite developed at Bend to visualize the data and act on it. “Our high-density datasets, which allow us to train a predictive model, differentiate our approach to process development.”
Eventually the goal is to use forecast results to control processes in real time.
Control over glycosylation is critical for maintaining homogeneity during production and across scales. Since glycoforms dictate pharmacokinetics their synthetic regulation amounts to quality control. The degree of homogeneity and similarity to innovator molecules is of particular interest to developers of biosimilars, who must demonstrate not only therapeutic equivalence but chemical similarity. Bioprocessors recognize this within the context that almost any factor may affect glycosylation patterns, including scale, a plant’s location, and raw material variability.

Bend Research, part of Capsugel Dosage Form Solutions, has developed Modular, Automated Sampling Technology (MAST™) for real-time, aseptic sampling. MAST is shown here with Bend’s SP200 sample pilot module.
Watch Those Ingredients
Variability in glycosylation patterns can arise from well-known factors like process variability, to subtle changes in impurity profiles in raw ingredients. Martin Gawlitzek, Ph.D., a scientist in late stage cell culture bioprocess development at Genentech, observed such a shift during process development for a CHO-derived humanized monoclonal antibody. He picked up the variability through capillary electrophoresis with laser-induced fluorescence detection.
Dr. Gawlitzek attributed the shift to a change in raw materials, specifically ferrous sulfate. Manganese, an impurity in ferrous sulfate, serves as a cofactor for certain enzymes involved in glycosylation. “Manganese plus-two [Mn2+] is a known co-factor for several glycosyltransferases in mammalian cells, including N-acetylglucosaminyl-transferase and β-1,4-galactosyl-transferase,” informs Dr. Gawlitzek. “It is not a co-factor for all glycosyltransferases.”
Some cell lines are more susceptible to concentration changes in manganese and even iron, and therefore at greater risk for larger shifts and greater glycan variability. “The magnitude of change is cell-line specific,” Dr. Gawlitzek concludes, and “constant manganese levels will likely improve glycosylation consistency.”
Dr. Gawlitzek’s solution was to increase manganese concentration in culture media to a level above the impurity range inherent in ferrous sulfate raw materials. Doing so swamps the effects of manganese impurities with a concentration above the effect threshold for manganese-dependent enzymes. This effected a consistent manganese effect regardless of what the impurity manganese concentrations. “The idea is to supplement the cell culture medium with manganese at a concentration that makes varying manganese impurity levels insignificant,” explains Dr. Gawlitzek.
In bioprocessing, glycosylation consistency may, within limits, be a greater indicator of quality than specific glycan patterns. That is certainly true here, given the variability arising from an uncontrollable factor—manganese impurity in ferrous sulfate. So while some intermediate manganese levels may have improved some quality attributes, achieving those levels could not be guaranteed.
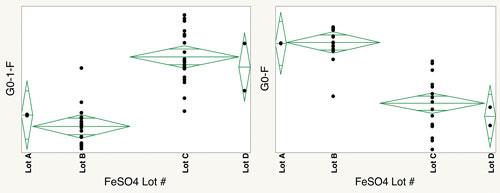
At Genentech, a variation in glycosylation patterns was uncovered during cell culture development and attributed to a change in raw materials, specifically ferrous sulfate, which may have different levels of manganese as an impurity. Shown here is the effect of different FeSO4 lots on N-glycan distribution (G0-1-F and G0-F).
Something to Be Said for Speed
“One cannot, in a short period of time, determine the specific physiological needs of CHO cells because all clonal cell lines are mutants or quasi-species,” explains Florian Wurm, Ph.D., chief scientist, Excellgene. “But if one has the right high-throughput approach for culture medium and process optimization, one may find the right match between cell and medium/feed/process.
Excellgene uses an optimized host cell line, CHOExpress™, with a doubling time of less than 14 hours and cell densities of 1,014 cells per mL in batch culture. CHOExpress also features a unique transposon-based expression technology that mediates integration into active chromatin. Together, these technologies enable production of more than 200 mg/L of expressed protein at the pool stage.
“A slow-growing host system is not well suited to rapid cell-line development. Everything takes more time,” Dr Wurm notes. “A small-scale system with high-throughput character, however, can be highly predictive of large-scale performance. We can run 1,000 bioreactors simultaneously.”
The bottom line, according to Excellgene, is the generation of stable cell lines in 12 weeks and the development of high-yield, multigram-per-liter processes, including stability assessment, in eight months—at least three to four months less time than conventional cell-line/process development.
Improving Glycan Analysis for Therapeutic Antibodies
During bioproduction of a therapeutic monoclonal antibody, a costly tradeoff exists in the QA procedures that ensure the biomolecule’s glycan composition remains within strict FDA-approved specifications. Accurate N-glycan profiles often require incubations too lengthy to allow remediation of quality issues during ongoing fermentation and purification, but truncated incubations risk failing to detect out-of-specification lots.
According to Alicia Bielik, Ph.D., a glycobiology product manager at New England BioLabs (NEB), the company has developed a novel glycosidase, available as Rapid PNGase F, for its ability to completely remove all N-linked oligosaccharides on antibodies and fusion proteins in reactions shorter than 10 minutes.
“Testing on rituximab, etanercept, and cetuximab showed that complete deglycosylation is accomplished after a five minute incubation at 50°C,” said Dr. Bielik. “Cetuximab, known to have a recalcitrant Fab N-glycan, required an additional two minute pre-incubation at 80°C. In contrast, standard PNGase F can not completely deglycosylate these therapeutic antibodies after a one hour treatment.”
Completion of deglycosylation was determined by analysis of released N-glycans as well as ESI-TOF analysis of the intact protein, added Paula Magnelli, Ph.D., a research scientist at the company. N-glycans were labeled by reductive amination and, after SPE-HILIC cleanup, were analyzed by LC-MS.
“The intact heavy chain of the deglycosylated antibody was analyzed by ESI-TOF after buffer exchange, confirming that N-glycan removal reached completion without bias,” continued Dr. Magnelli. “The data were reproducible with negligible variation, including for low-abundance species. All major and minor glycans previously reported in the literature were detected, including the species corresponding to cetuximab’s Fab domain.”
“The data demonstrate potential to align regulatory and rapid quality control testing, as well as present an opportunity for a defensive intellectual property strategy, through precision glycan profiling, for developers of innovative biotherapeutics,” noted Dr. Magnelli.



