June 1, 2013 (Vol. 33, No. 11)
Sridhar Nadamuni
Innovations in instrumentation and application of mass spectrometry offer solutions to routine analytical challenges across a range of areas including glycoproteomics, homeland security, forensics, environmental analysis, and the food industry, among others.
Hydrophilic interaction liquid chromatography (HILIC) provides a different selectivity in HPLC compared to the classical reversed phase materials, especially for polar compounds. However, the increased need for mobile phases with improved MS compatibility has resulted in the widespread use of LC/MS instruments in analytical labs.
“One approach to address the demand for higher separation efficiency and increased sensitivity in HPLC is the development of the Fused-Core™ particle technology,” says Frank Michel, Ph.D., analytical & chromatography technology specialist Sigma-Aldrich Chemie. According to Dr. Michel, the LC/MS sensitivity can be increased via:
- Improved volatility of the usual mobile phases in HILIC
- High volatility of acetonitrile-rich eluent improves ionization
- Depending on compound class, sensitivity can be higher up to a factor of 100
Based on Dr. Michel’s conclusions, the new Fused-Core particle technology is associated with:
- Narrower peaks leading to decreased Limit of Detection (LOD)/Limit of Quantitation (LOQ)
- Higher efficiency on any HPLC system
- Shorter analysis times for continued efficiency
- Strength and durability suggested by 5 µm columns
The history of HPLC particle design is characterized by irregular, difficult-to-pack, clogging, not-very-robust materials compared to the current state-of-the-art totally porous format, says Dr. Michel. Fused-Core is an advanced new particle technology that now provides higher separation efficiency, he claims. “In a long HPLC column, e.g., 150 mm, this can be used for better separation and better sensitivity.”
This particle technology enables higher efficiency in HPLC leading to narrower, higher peaks. As a rule of thumb, the efficiency and sensitivity double when changing a method from a 3 μm material to a Fused-Core 2.7 μm material while the backpressure stays the same. “In a short HPLC column this can be used for shorter analysis time and higher throughput while maintaining the separation efficiency known from fully porous particles,” he says. The shorter diffusion pathway facilitates the mass transfer.
A combination of HILIC with Fused-Core particle technology results in ultrasensitive efficiencies, according to Dr. Michel. The current selection of stationary phases consists of eight different chemistries. “As selectivity is the key to success in HPLC method development, in the future, the portfolio of different stationary phases will be expanded to facilitate method development in HPLC and in HILIC,” he explains.
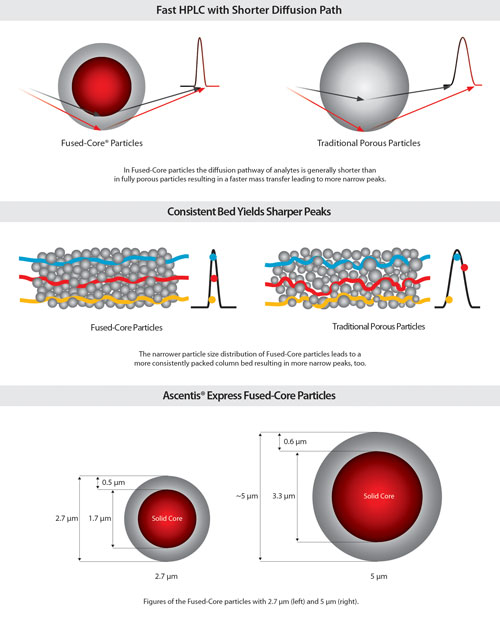
Sigma-Aldrich reports that its particle technology enables higher efficiency. (A) In Fused-Core particles the diffusion pathways of analytes is generally shorter than in fully porous particles. (B) The narrower particle size distribution of Fused-Core particles leads to a more consistently packed column bed, resulting in narrower peaks. (C) Fused-Core particles with 2.7 µm (left), 5 µm (right).
Chip-Based FAIMS Coupled to MS
High-field asymmetric waveform ion mobility spectrometry (FAIMS) uses a variation of ion drift velocity in atmospheric pressure environments at very high electric fields. It is often seen as a candidate for enhancing the separation provided by liquid chromatography (LC), or even potentially replacing the LC stage entirely for some applications.
Applications of FAIMS extend from homeland security to environmental analysis to proteomics. Interfaced with electrospray ionization, FAIMS serves as an additional separation mode between liquid chromatography and mass spectrometry in proteomic studies.
As FAIMS separation is orthogonal to both LC and MS, it is used as a means of on-line fractionation to improve the detection of peptides in complex samples. By filtering out chemical noise, FAIMS has been shown to improve the active range and the detection limits of ions. It separates isobaric ions, including diastereoisomers, and isotopes.
Reducing background ions by isolating ions of interest, FAIMS simplifies spectra of complex mixtures by resolving the mixture into a series of simpler subsets of ions. In fact, by adding solvent vapor to the carrier gas, FAIMS is also a potential greener bioanalytical technique, compared with traditional HPLC methodologies, as it uses an alternative method for separation and reduces solvent use.
Researchers have successfully used the tool in the analysis of glycosylation, with potential applications in the field of glycoproteomics. They have used FAIMS to separate localization isomers of O-linked glycopeptides. The technique has applications in diagnostics, e.g., a decrease in O-linked glycosylation of microtubule-associated protein tau has been shown in patients with Alzheimer’s disease. It can remove interfering ion species and can select peptide charge states optimal for identification by tandem MS.
A disadvantage of all differential mobility devices has been the scanning duty cycle relative to fast LC. It has been a challenge to achieve performance that allows simultaneous effective separation, high transmission, and scan speed. Robin Philp, Ph.D., LC/MS manager at Agilent Technologies, Singapore, notes a new generation of microscale ion-mobility devices that he says enable this ability and could improve high-throughput multidimensional separations.
Lester Taylor, Ph.D., the director of LC/MS product marketing at Agilent Technologies, explained that researchers at Agilent in partnership with colleagues at Owlstone Nanotech have developed a chip-based technology for LC/MS application based on Owlstone’s FAIMS filter as a front-end separation module for Agilent’s time-of-flight mass spectrometers (TOF-MS).
“The goal is to introduce a third means of separation based on ion mobility, in addition to the HPLC and mass correlation,” according to Dr. Taylor. The ion-mobility device from Agilent is extremely compact, about the size of a U.S. quarter, as Dr. Taylor explains. Several mass spectrometry applications are expected to benefit from high-speed preseparation. For instance, “we can look at different charge states (single-, double-, or triple-charged species), in the analysis of peptides in proteomics; we are able to tune the device to selectively transmit either +, 2+, or 3+ charges.”
Basically, the device can be used to select the number of charges of ion species, thereby helping to reduce the complexity of the peptide mixture, and isolate the specific peptide of interest, without contamination from overlapping or extraneous species that happen to be transmitted at a given mass, according to Dr. Taylor. By introducing a modifier gas to enhance ion-mobility separation, researchers have been able to separate morphine from its metabolites such as morphine glucuronide.
The chip-based ion mobility device has also been applied in removing chemical interference in plasma, with drugs at low limits of detection. In the future, the technology will help simplify analysis of complex samples by adding another selectivity dimension, according to Dr. Taylor. The goal is to simplify sample analysis. Agilent’s ultra-fast chip-based ion mobility separation coupled to mass spectrometry will be formally introduced this month.
In addition to innovative analytical applications in drug discovery and development, effective analytical protocols that can be routinely used to authenticate either plant extracts or commercial products with therapeutic potential are needed.
Forensics
Recent advances at Thermo Fisher Scientific involving software-assisted and automated approaches for chromatography and mass spectrometry (MS) have promising new applications in the area of forensic medicine. Time is of the essence in the analysis of forensic results (for example, in the aftermath of the Boston Marathon bombings on April 15, 2013).
“Time is especially important in the forensic industry where reliability of sample chain of custody is pretty much the name of the game, along with a full and accurate chain of sample custody, which may be used in a court of law,” says Sarah Perry, principal consultant for LIMS Logical Consultancy. “In my experience, mass spectrometry, chromatography, and informatics solutions help customers perform highly accurate quantitative and qualitative analysis, as we know in forensics,” says Perry.
Laboratories routinely handle sensitive samples that may otherwise run the risk of degradation, if not properly collected, stored, and archived prior to testing. In order to provide timely analysis of results, forensic labs require the automation of laboratory processes where possible.
The automated integration of instrument data has become essential. According to Perry, the automation is facilitated by “the use of LIMS to track all sample processing and continuity data and manage workflow for instrumentation, coupled with scientific data management system (SDMS) software such as Thermo Scientific DataManager, which is a vendor-agnostic application.” The SDMS software allows for the collection, packaging, and archiving of raw instrument data and the ability to link the data to the relevant LIMS record, as Perry explained.
The use of an SDMS such as DataManager allows collection of raw instrument data from any instrument and then sharing of that data with other users in their format of choice. Perry commented that “the raw data can now be viewed over any mobile device, such as a notepad or smartphone, enabling the forensic scientist to have access to critical data while they’re in the lab or in the field.”
Integration of the instruments with the LIMS (for example, mass spectrometry, chromatography, Electronic Lab Notebook) saves time and costs for the lab personnel by eliminating manual errors. It enhances the reliability of data, easy retrieval, and submission for regulatory or legal purposes.
“To me, automated instrument data management includes not just the handling of the raw data produced by the instrument at the end of a process, but also the data required by the instrument to allow it to run and accurately link the results obtained to the samples processed.”
In a paperless environment, especially in the regulated industries, the LIMS serves as the central repository for all instrument data, allowing for more complete automation of the lab. The emphasis is more on “stable, leading edge” rather than “bleeding edge” applications, in Perry’s view.

According to Thermo Scientific, use of its LIMS allows a forensic lab to track all sample processing for chain of custody and complete audit trails. It also provides instrument integration and an automated workflow for improved lab efficiency.
Chemical Fingerprinting
The limited availability of Hoodia species and its increasing popularity as a weight loss product have led to unscrupulous practices with contamination by other species or even genera. In light of the serious concerns related to the safety of counterfeit commercial products sold as Hoodia, researchers led by Kate Yu, Ph.D., senior manager, pharmaceutical & life science at Waters, have developed a generic UltraPerformance LC® (UPLC®)/MS workflow for the authentication of Hoodia gardonii based on comprehensive chemical fingerprinting.
“We have developed a holistic and solid approach, based on comprehensive profiling, digging deep to come up with a relatively simple solution for Hoodia analysis,” says Dr. Yu. She and her colleagues used the UPLC/Qtof MSE/Multivariate Statistical Analysis and the POSI±IVE Exact Mass Screening Analysis to quickly analyze plant extracts or dietary supplement samples and generate quantitative reports as well as detect any adulteration or artificial spiking of the samples. The POSI±IVE technology facilitates screening and quantitation in a single automated workflow, she says.
Indeed, novel LC/MS techniques analyzing microdose and other low-level clinical studies for metabolites and drug-related material are poised to significantly change the analytical approach to industrial applications.

Waters has developed a mass spec workflow for the authentication of Hoodia gardonii, which is increasingly used as a weight loss product, based on comprehensive chemical fingerprinting.







