April 1, 2017 (Vol. 37, No. 7)
MaryAnn Labant
If You’re Up Against Cancer, Don’t Fight Fair. The Name of The Game Is Combination Therapy
A growing recognition that both the innate and adaptive immune systems can play critical roles in antitumor activity continues to fuel activity in immuno-oncology research. As new approaches emerge and enter clinical trials, the search continues to find ways to harness the body’s natural defenses in the fight against cancer.
Across all approaches, combination therapy has emerged as the most promising concept, although it is still too costly for broad use.
The CD47 protein is found on the surface of many cells. The body uses it to mark cells that should be protected, distinguishing them from aged or diseased cells that should be eliminated. For example, young red blood cells start off with a lot of CD47 on their cell surface but slowly lose it as they age. At some point, the amount of CD47 on the surface is insufficient to stave off the macrophages. Older cells are devoured and destroyed, making way for new red blood cells.
Unfortunately, some cells that should be destroyed are not. Researchers at Stanford University have discovered that nearly every kind of cancer cell has a large amount of CD47 on the cell surface. This protein signal protects the cancer against attack by the body’s immune system. Animal models demonstrate that if the CD47 protective signal is blocked through the use of anti-CD47 antibodies, macrophages will consume and destroy cancer cells.
Blocking CD47 can have the effect of turning off a “don’t eat me” signal, favoring phagocytosis. Usually this kind of blocking is accomplished with an antibody, but there are alternatives. For example, TTI-621 is a checkpoint inhibitor that can target CD47. Currently being developed by Trillium Therapeutics, TTI-621 is a fusion protein that is structurally distinct from CD47-blocking antibodies. It consists of the natural ligand for CD47, SIRPα, linked to a human IgG1 Fc region.
TTI-621 is a dual-function molecule: the SIRPα domain blocks CD47, and the IgG1 Fc delivers an “eat” signal through Fc gamma receptors (FcgRs). The molecule not only triggers macrophage phagocytosis, it also increases tumor antigen presentation and prompts an augmented tumor-specific T-cell response. These results support a model that impacts both the innate and adaptive immune systems.
According to Robert Uger, Ph.D., Trillium Therapeutics’ chief science officer, the structure of TTI-621 confers two important benefits. First, TTI-621 does not appreciably bind human erythrocytes, is unlikely to cause anemia in patients, and likely avoids the large antigen “sink” that is created by erythrocyte-expressed CD47. Second, the IgG1 Fc increases the potency of the molecule compared to agents that simply block the CD47 “do not eat” signal.
TTI-621 is being developed to target blood cancers as well as for the treatment of solid tumors. A Phase I study of intravenously administered TTI-621 in patients with advanced hematologic malignancies was initiated a year ago.
Trillium Therapeutics recently launched a multicenter Phase I clinical study evaluating intratumoral administration of TTI-621 in patients with relapsed or refractory percutaneously accessible cancers. Both monotherapy and combination therapy strategies are being pursued.
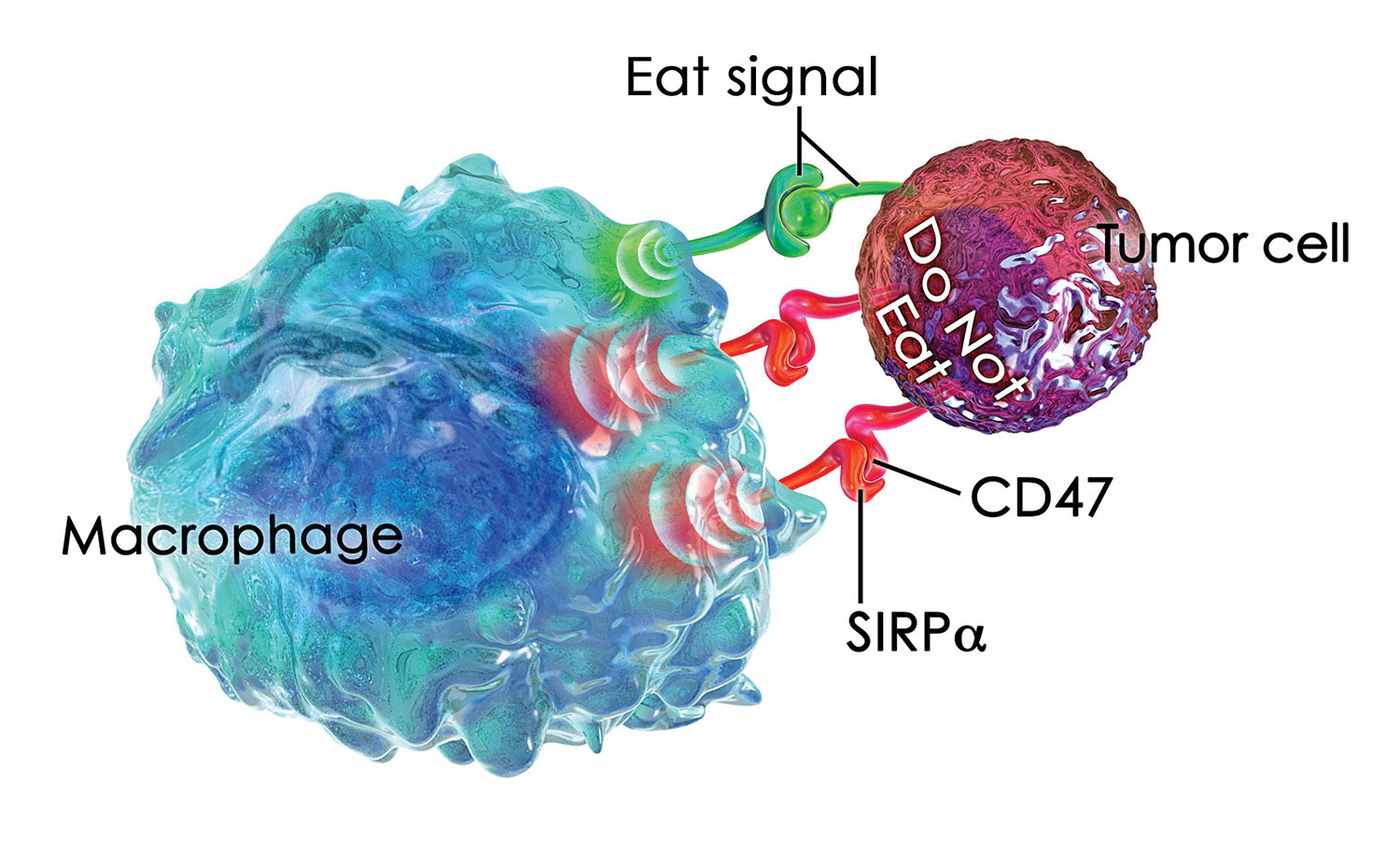
Trillium Therapeutics’ TTI-621, a SIRPaFc fusion protein, targets CD47, a molecule that is expressed at high levels by many different types of cancer cells. When CD47 binds to SIRPa on macrophages, it delivers a “do not eat” signal that inhibits the ability of macrophages to engulf and destroy malignant cells.
Insight from Other Disciplines
To inform its immuno-oncology development efforts, OSE Immunotherapeutics studies the ability of the body’s organs to promote immunity and tolerance. In particular, the company uses a think-tank approach to examine how this ability is understood in different medical contexts, such as transplantation, infectious disease, and pregnancy.
Insights from organ transplantation studies helped OSE Immunotherapeutics develop Effi-dem, an anti-SIRPα antagonist IgG4 monoclonal antibody. In a kidney transplant rodent model, myeloid-derived suppressor cells (MDSCs) that were overexpressing SIRPα were found to be responsible for tolerance. Infusing Effi-dem into a transplant model caused organ rejection by awakening the immune system. T cells developed, and macrophages were activated.
These findings led to the hypothesis that a SIRPα antagonist could improve the potential rejection of a tumor by promoting collaboration between macrophages and T cells. Antagonistic SIRPα antibodies compete with CD47, a counter-ligand.
Inflammatory tumors are infiltrated by myeloid cells. The information generated by the tumor gives a signal for myeloid cells to grow and locate inside the tumor, where they create a microenvironment that blocks the immune system. The maturity of the myeloid cells is controlled by SIRPα and is associated with an immune-suppressive activity. Effi-dem acts as a brake by targeting SIRPα on myeloid cells.
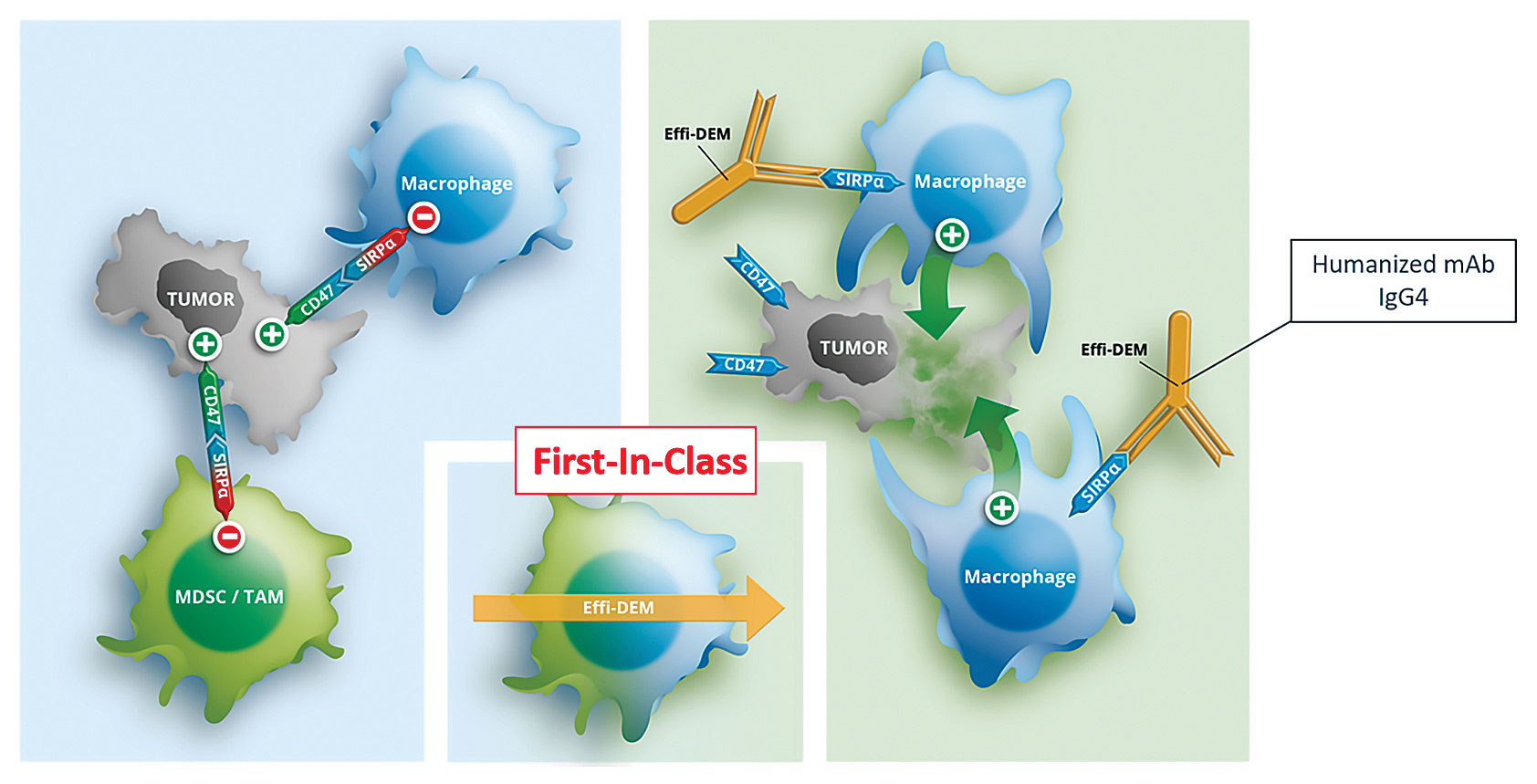
Effi-dem, a monoclonal antibody from OSE Immunotherapeutics, blocks the SIRPa receptor on myeloid-derived suppressor cells (MDSCs) and tumor-associated macrophages (TAMs), converting these tumor-infiltrating cells into nonsuppressive effector cells, allowing for tumor destruction and a rescue of the immune response.
“We want to enable the immune system to fight the tumor,” explains Bernard Vanhove, Ph.D., COO at OSE Immunotherapeutics. “Effi-dem acts only on the myeloid cells that are infiltrating the tumor and blocking the immune response. It does not act on the tumor cells. This is quite different than other CD47 approaches.”
Cancer cells overexpress CD47, a receptor for the inhibitor domain of SIRPα, to protect themselves. SIRPα has a more restricted expression; it is expressed only by certain types of myeloid cells. Effi-dem targets a very specific subtype of leukocytes, MDSCs and tumor-associated macrophages (TAMs), in the tumor microenvironment.
Effi-dem interrupts the molecular dialogue between SIRPα-expressing cells and CD47-expressing cells that inhibits the phagocytic activity of the macrophages; the more restrictive targeting results in less toxicity and fewer side effects, such as anemia.
“We are currently selecting a series of cancers where SIRPα and other myeloid cell markers are highly expressed in the microenvironment,” continues Dr. Vanhove. “Animal models are predicting an immune response against the tumors. Once treated, they reject implanted tumors because T cells get awakened and become able to fight against the tumor.”
The hope is Effi-dem will provide long-term immunization and protection against metastasis. Effi-dem is planned as both a monotherapy and also a combination therapeutic.
Animal models are used to predict an immune response against tumors. Once treated, implanted tumors are rejected because T cells get awakened and become able to fight against the tumor. [Royaltystockphotos/POBA /Getty Images]
Designing Cell Engagers
Malignant cells have acquired mechanisms to evade immune cell recognition. In principle, then, all approaches supporting immune cell-based elimination of tumor cells are of therapeutic value. Immune evasion affects many different immune cells; thus, therapeutically successful strategies ideally include innate cells, such as natural killer (NK) cells and macrophages, and elements of adaptive immunity, such as T cells.
Building on its proprietary platforms, Affimed develops biologics that can be optimized (in terms of affinity, avidity, and specificity) and reliably manufactured. The company has characterized and developed a solid pipeline of both NK-cell and T-cell engagers and brought a lead candidate forward into clinical development.
AFM13 is a bispecific NK-cell engager that targets CD16A on NK cells and CD30 on tumor cells. The antibody is tetravalent with four binding sites, two that bind to the NK cell and two that attach to the target tumor cell. Binding to CD16A, AFM13 specifically activates and modulates the NK cell by establishing a strong bridge to the tumor cell. This triggers a signal cascade that leads to the destruction of the tumor cell.
AFM13 is designed to treat CD30-positive malignancies including Hodgkin’s lymphoma (HL) and T-cell lymphoma (TCL) and is currently in Phase II studies in HL patients. Based on its favorable safety profile, AFM13 is being developed both as a monotherapy and as an element in combination therapeutics.
“Our NK-cell engager AFM13 overcomes immune evasion by educating NK cells to recognize tumor cells, thus initiating the elimination process,” explains Martin Treder, Ph.D., Affimed’s CSO. “Based on preclinical data that shows immune cross-talk, we believe that engaging NK cells may offer the unique opportunity to activate both innate and adaptive immunity.”
This could ultimately lead to deeper responses and potentially even cures. Affimed is currently investigating the potential activation of macrophages mediated through its NK-cell engaging technology.
A clinical study of AFM13 and the anti-PD-1 antibody Keytruda is ongoing in severely ill HL patients to investigate the attack on cancer cells by both innate and adaptive immune systems. Another recently announced collaboration is with adoptive NK cells and the MD Anderson Cancer Center.
Predicting In Vitro Potency
Potency analyses in animal models can be time consuming, and many traditional in vitro cell-killing assays suffer from low signal-to-noise ratios and poor reproducibility. A challenge in immuno-oncology is developing in vitro potency assays that provide actionable results by accurately predicting in vivo therapeutic behavior.
“The killing of a target cell by a given immunotherapy is a multifaceted process involving a large number of events,” states Yama Abassi, Ph.D., vice president of global strategic business development, ACEA Biosciences. “Although numerous assays exist for monitoring these phenomena in a reductionistic manner, many of these biochemical approaches are poorly predictive of cell-killing efficacy.”
ACEA’s xCELLigence Real-Time Cell Analysis (RTCA) instruments utilize gold electrodes embedded in the bottom of a patented microtiter plate wells to noninvasively monitor cell status. When target cancer cells adhere to the bottom of the plate, they act as insulators, making it more difficult for a miniscule electric current to flow between electrodes.
This impedance signal provides an extremely sensitive assessment of cell number, size, and attachment quality. Immune effector cells are not naturally adherent. They do not contribute to the impedance signal, making it possible to selectively monitor target cell health and behavior during a heterogeneous cell-killing assay.
As immune effector cells, oncolytic viruses, checkpoint inhibitors, or various combination therapies induce target cell death, the xCELLigence instrument tracks and plots in real-time.
xCELLigence assays permit monitoring of immune cell-mediated killing even at physiologically relevant Effector:Target ratios as low as 0.1:1. Data points can be acquired every 10 seconds over the course of weeks, if desired.
The available 1 × 96- and 6 × 96-well formats allow side-by-side monitoring of a diverse array of constructs, conditions, and combination therapies. An immunotherapy-specific software package facilitates the design and organization of experimental parameters, and enables plotting of metrics such as the percentage of cytotoxicity, the half maximal effective concentration (EC50), and the half knockdown time (KT50).
Immunotherapy can achieve its full potential, Dr. Abassi believes, if it adopts a “bench to bedside” format, one in which therapies are optimized in ex vivo assays employing both a patient’s tumor and immune effector cells. ACEA’s two new kits enable a patient’s immune effector cells to be analyzed for potency against liquid tumor target cells that are selectively tethered to the impedance electrodes using antibodies.
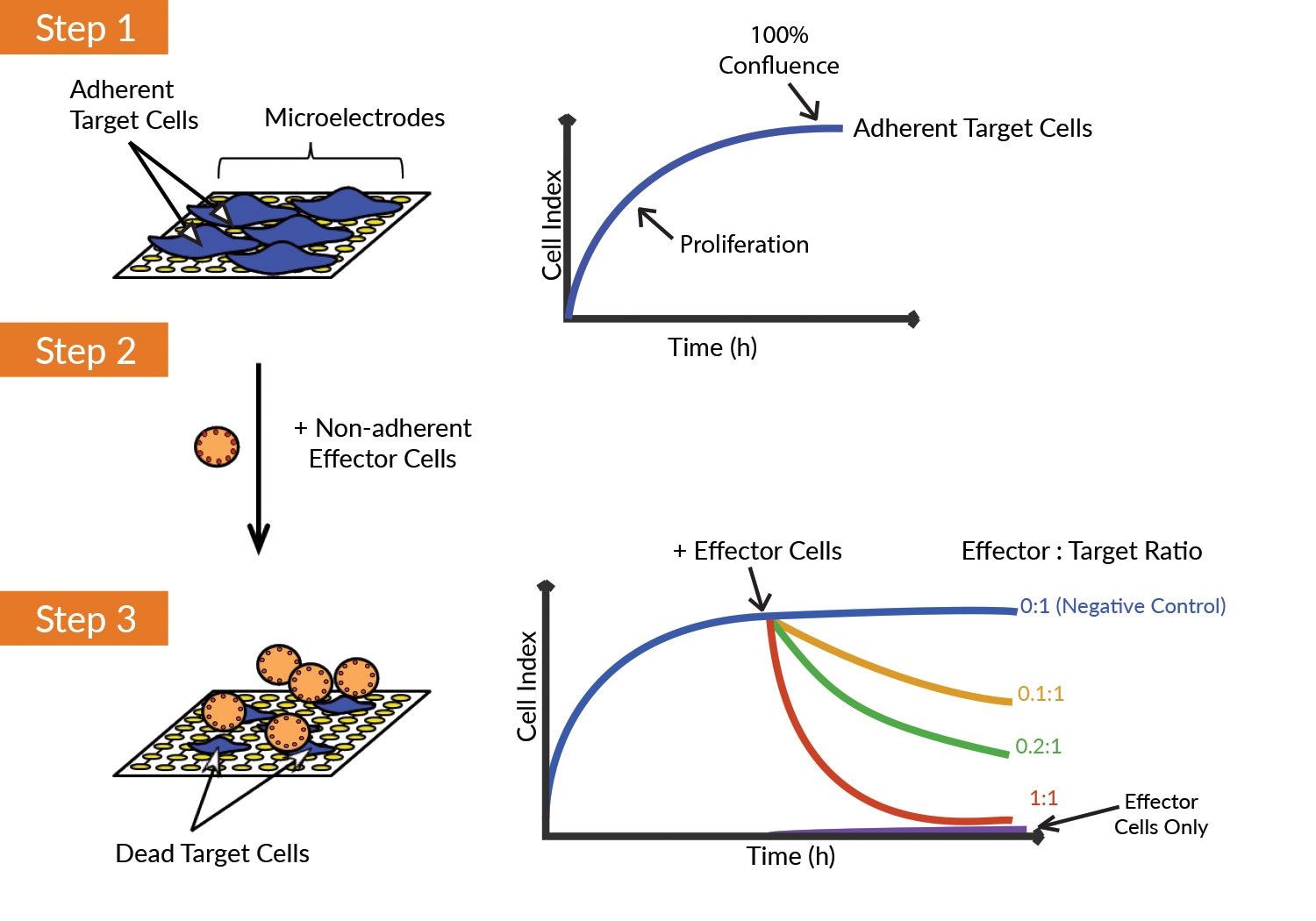
ACEA Biosciences’ xCELLigence instruments noninvasively monitor cell status. (1) Target (tumor) cells adhere to the wells of an electronic microtiter plate and impede the flow of an electric current. As cell density increases, impedance (or the “cell index”) increases. (2) Nonadherent effector (immune) cells do not directly alter impedance. (3) When immune cells destroy tumor cells, changes in cell number and attachment quality can be monitored in real time via the continuous acquisition of impedance data.
Clinical Endpoints in Immuno-Oncology
Overall survival (OS) remains the top measure of success for immuno-oncology (IO) interventions; but assessing it requires lengthy clinical trials, a challenge further compounded by the durable responses IO can convey. This has led to the development of surrogate endpoints, which allow developers to assess efficacy earlier. The faster time frame has led to more promising therapies moving to quicker review and approval by the FDA.
However, defining surrogate endpoints for immunotherapy trials is challenging. For example, applying traditional chemotherapy-based measures to immunotherapy can incorrectly attribute an apparent increase in tumor burden to disease progression (called “pseudoprogression”). This is because T-cell infiltration initially creates the illusion of tumor growth and disease progression that is difficult to confirm without a biopsy.
Standardizing Tumor Measurement
New measures beyond RECIST, such as irRECIST and EORTC’s newly announced iRECIST, look to address delayed responses after pseudoprogression, and to standardize tumor measurement and definitions for progression which can be used in immunotherapy clinical trials.
Immunotherapy endpoints can also differ depending on the phase of the clinical study. In early phase studies, primary endpoints are nearly always focused on safety, pharmacokinetics, and pharmacodynamics. Confirming the intended mechanism of action is central, as is identifying the dose level that offers the best balance of treatment and safety. Rather than a maximum tolerated dose (MTD), this is often a recommended Phase II dose (RP2D) based on measures that can include immune responses and biomarker target engagement.
“Additionally, early phase studies increasingly use efficacy endpoints such as overall response rate (ORR), duration of response (DoR), and progression-free survival (PFS) as secondary, or exploratory measures,” says Andrew Zupnick, Ph.D., vice president, oncology division, Novella Clinical.
Collecting data about treatment efficacy can aid in drug development, and especially pronounced effects may lead to a breakthrough therapy designation by the FDA.
Because of the benefits to clinical trial protocols that surrogate endpoints offer, the search will continue for surrogate endpoints that accurately predict overall survival, and ultimately replace survival rates entirely as a clinical trial’s defining metric. Even today, in late phase studies, surrogate endpoints commonly take priority and are regularly a source of accelerated approval by the FDA.
While there is great promise in store for surrogate endpoints, immunotherapy-specific assessments continue to evolve, and for the foreseeable future, Dr. Zupnick says, overall survival will remain the standard for clinical relevance.
Better Prediction of Immunotherapy Response
A vision for personalized immunotherapy can only be achieved by identifying the people most likely to respond to this important class of medicine. Predictive biomarkers are essential to this effort, but current methods have major drawbacks.
Measuring PD-L1 protein expression using immunohistochemistry, for example, is based on subjective evaluation and produces variable results between assays. This has had meaningful impacts on the design and outcomes of clinical trials.
Tumor mutational burden (TMB) is a new predictive clinical marker for cancer immunotherapies that could provide a more reliable and quantitative solution, according to scientists at Foundation Medicine. TMB represents the number of mutations within a tumor and has been shown to predict response to checkpoint inhibitor immunotherapies across several tumor types.
For example, in a recent study of advanced bladder cancer (Balar et al., Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial, Lancet. 2017;389:67-76), researchers found that TMB, as measured with the FoundationOne® comprehensive genomic profiling (CGP) assay, was a better predictor of response to anti-PD-L1 therapy than PD-L1 testing.
Predictive biomarkers need to keep pace with the science of precision medicine in order to maximize the value of immunotherapy. Measuring TMB with CGP assays is showing itself to be one potentially important way to do so.
Novel Pathology Imaging Platform for Studying Cancer
Innovative research has led to the discovery of many promising targeted anti-cancer therapies, but disease mechanisms are often complex, making it difficult for researchers to understand predictive biomarkers of the disease course and response to immunotherapy approaches.
New multiplexed IHC staining technology enables immuno-oncology and pathology researchers to gain a deeper understanding of disease mechanisms and addresses a critical need by revealing the cell-level biology occurring in the tumor and its microenvironment, which drives disease progression and response to immunotherapy.
Multiplexed IHC staining reveals cell types present in the tumor microenvironment, including tumor cells and various immune cells associated with natural and adaptive immune responses. It also reveals the functional states of cells and whether or not the cells are expressing checkpoint receptors and ligands associated with immune evasion.
Once cells are detected and their functional states determined, cell-to-cell biology can be assessed by characterizing the spatial distributions of these cells, especially relative to the interface between the tumor and the microenvironment.
PerkinElmer’s Vectra® Polaris™ Automated Quantitative Pathology Imaging System, launched in January, is a slide analysis platform developed specifically to analyze multiplexed IHC via a high-throughput, rugged, high-speed slide analysis research system, according to company officials. It provides both digital pathology whole slide scanning in both brightfield and fluorescence applications, plus capability to overlay PerkinElmer’s multispectral technology to probe areas of interest more deeply with higher multiplexing, explains Cliff Hoyt, oncology fellow, discovery and analytical solutions, PerkinElmer.
“Vectra Polaris is the latest addition to PerkinElmer’s end-to-end workflow called Phenoptics, which starts with multiplexed IHC staining using tyramide signal amplification, proceeds to scanning and imaging on the Vectra Polaris, then goes on to analysis using machine learning-based pattern-recognition software to detect areas of tumor and microenvironment and individual cells,” he says.
The image analysis process ends with cell “phenotyping” to fully capture the cellular interactions occurring in situ within in the tumor, adds Hoyt.



