April 15, 2009 (Vol. 29, No. 8)
Andreas Schneider
Meticulous Attention, Constant Revision, Supervision, and Assistance Are Essential
The FDA’s Quality by Design (QbD) initiative, part of the larger Process Analytical Technology (PAT) effort, will require an enormous undertaking by companies that plan to employ its principles. The goal of QbD is for companies to consistently and quickly produce high-quality products by demonstrating a deep scientific understanding of their own manufacturing processes.
A manufacturing philosophy like this would greatly reduce the need for heavy regulatory oversight. To get ta point where the benefits of such a systemic overhaul would prove beneficial, however, many companies must spend years documenting and gathering validated processes.
The ideal QbD set-up for manufacturing biopharmaceuticals is a sophisticated, complicated affair. Every component must work to facilitate automation in one way or another. The first major piece in a system with automated cell culture analysis should be a bioreactor with a manufacturing execution system (MES) or laboratory information management system (LIMS) that can automatically adjust processes based on the measurement data of critical process parameters.
The culturing vessel can either be a steel tank, a glass vessel, or a single-use bag. All along the way, in-line sensors of all types are set up to measure pH, pressure, temperature, and CO2, to name a few factors. A key enabling product is Bayer Technology Services’ new sterile online sampling unit, which automatically removes samples from a bioreactor and transfers them to a liquid-management unit.
Sterilization Methods
Right now, there are two sterilization methods available: chemical and steam. The advantage of chemical sterilization is that there is no need for additional infrastructure, i.e., a steam connection in the lab. Smaller-scale process-development laboratories typically do not have steam readily available. The main disadvantage is that the contamination risk is higher than with steam.
The sterile barrier in the process chain is critical, and the only proven technology to keep ports sterile is steam sterilization. In most cases, however, the implications of contamination are minor in small-volume bioreactors since the value of a potential loss of a batch is low and parallel fermentation processes are run simultaneously anyway.
Steam sterilization is the most common and established technology used to sample a culture from a bioreactor. The advantages of steam sterilization begin with its reputation for robust and reliable performance, ideally suited for larger bioreactors. It has been proven to be quick, clean, and consistent.
Steam sterilization in automated sampling platforms is not without its share of vulnerabilities, however. Since the cell culture sample transport tube, part of the liquid-handling component of this automated system, has to be connected to the online sampling port, there is a risk of cross-contamination.
Also, as mentioned previously, a steam supply would clearly be required, a potentially expensive proposition. There are new developments in the lab-equipment industry, however, where offline steam supply units can provide steam without additional infrastructure in the lab (Figure 1).
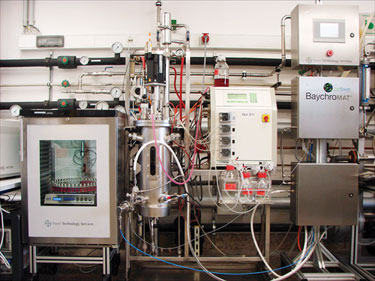
Figure 1. BaychroMAT Cell Count, at the Bayer Technology Services pilot plant, is a fully operational QbD system construct.
Liquid Handling
The second major piece to the QbD puzzle is a liquid-handling system that does all sample-preparation steps in a fully automated fashion. This includes splitting samples (to feed multiple supply lines), filtration steps, and transportation to cell culture analyzers. It is important at this stage, for accurate measurement, that the cell culture sample be transferred from the sample port to the analyzers without losing volume in the tube or causing damage to cells, no matter the distance.
Analyzers
Another important part of the puzzle is the analyzers themselves. Analyzer platforms that run measurements of various parameters in a fully automated, reliable, and reproducible manner are vital for accuracy and cGMP compliance. Each analyzer must be maintenance-free during the fermentation process, cleaning procedures, and calibration routines and must also run fully automated and be traceable. That is why cell culture analyzers that rely upon membrane technology are not compatible with a fully automated manufacturing process, as the membranes require regular maintenance.
The final puzzle piece is data-sharing capabilities. All analyzer measurement results, in-line and on-line, must then be transferred electronically and automatically to a central database where they can be reviewed and/or forwarded to MES, LIMS, and/or historian data-management systems. A historian data system compares the measurement data of the current batch with past batches or golden batches (ideal batches).
Its simulation software predicts future process values based on the historian data, current batch data, and algorithms predicated on the correlation between all critical process parameters. Because critical process parameter correlations are based on past evidence, as well as scientifically sound models, the predicted values can be used to calculate any adjustments that need to be made in order to improve the quality of a cell culture product, or at least lead the given culturing process in the right direction.
In order to even have reliable measurement data, the process and the analyzers must all be synchronized, i.e., unit-to-unit comparability for the analysis equipment involved is necessary. This is one of those situations where it can be important to use equipment from one manufacturer. It is frustrating to run a process simulation or prediction if it is not known whether the measurement results of past processes are comparable to the current batch.
Innovatis, a manufacturer of cell culture analysis equipment, has partnered with Bayer Technology Services to create BaychroMAT, a fully functional online sampling and online analytic system. Cedex and CuBiAn, also from innovatis, are cell counters and media analyzers, respectively, that have been specifically designed with QbD in mind (Figures 2 and 3). They possess rapid data-sharing capabilities and work in tandem with BaychroMAT to provide a fully automated cell culture analysis system that is open and modular enough to adapt to any process.
The BaychroMAT system can be incorporated into any cell culture analyzer, however, completely automated data transfer is not possible without Cedex and CuBiAn. The collected data from Cedex and CuBiAn is used with custom algorithms to calculate the process adjustments. Those calculated adjustments are then sent by the LIMS or MES to the control software of the bioreactor, and the required adjustments are done automatically.
Although these process adjustments are all fully automated, the software will readily display the automated decisions being made for review by personnel. The software later reviews all adjustments to ensure that the expected measurement value and/or product quality has been reached.

Figure 2. Cedex and CuBiAn are maintenance-free cell counters and media analyzers, respectively, which are fully integrated into BaychroMAT Cell Count systems.

Figure 3. CuBiAn
Benefits
The rewards of QbD in a biopharmaceutical setting are immense. Because a product’s cost runs parallel to the amount of risk associated with it (safety and efficacy), the removal of risk early on in a process will lower the final cost of manufacturing. The ultimate goal is to try to remove a lot, if not most, of the legendary unpredictability and risk associated with biomanufacturing processes.
Implementing such a system doesn’t need to be daunting. Innovatis can assist in the difficult process of evaluating and executing implementation strategies. The key is to define the numerous CQAs (critical quality attributes) of the product, which are essentially risk assessments, prioritize them, and then monitor them.
CQA determination is an evolving practice throughout the entire drug development and manufacturing process. As new information is gathered about a product and its characteristics, CQAs must also be continuously challenged and reassessed. The process parameters that have a critical impact on CQAs must be identified.
These critical parameters have to be monitored in real-time in order to find correlations for process adjustments and to evaluate their impact. Since there are a wide range of data sets, thousands of measurement points, and CQAs that must be constantly reevaluated, it is clear that the fundamental groundwork to even begin QbD implementation is substantial.
Andreas Schneider (andreas.schneider @innovatis.com) is business
development manager at innovatis.
Web: www.innovatis.com.