December 1, 2010 (Vol. 30, No. 21)
Nina Flanagan
NCI Spearheading Development of Standardized Guidelines to Improve Operations
Researchers in academia and industry realize the importance of biospecimens not only for diagnostics but also for drug discovery and development. Unfortunately, the collection and processing of these precious assets varies from lab to lab, raising doubts about the accuracy of research data. IIR’s “Biorepositories” conference, held in Boston recently, provided information on new efforts to enhance biorepository operations and optimize biospecimen integrity.
Economic pressures are forcing companies to focus more on their biospecimen collection and to re-evaluate its management. “Pharmaceutical firms are realizing that it’s difficult to leverage the value of scattered resources,” said Cathy Michael, global head of sample management at BioStorage Technologies.

BioStorage Technologies has designed a mobile unit specifically for sample transition, relocation, and emergency response services. Equipped with ultralow freezer and refrigeration systems, the 52-foot, multitemperature trailer is capable of transporting many different kinds of cargo, including biomaterials such as plasma, blood, and tissue samples.
Standards
Another challenge is the question of future standards. “Activity now is sometimes constrained by an unknown future need,” she added. Michael presented information on applying conventional wisdom in innovative ways to maintain maximum efficiency for biorepository operations.
She also talked about relocation of samples and utilizing assets as a renewable sample resource. Many companies have scattered resources, she reported, and conventional wisdom says this is okay, because samples are close to the scientists at the bench. An emerging approach is to consolidate samples as a full asset collection and to catalog it, allowing scientists to make time-sensitive decisions based on annotated information (patient age, co-drugs, co-morbidity, etc.). This allows a collection to be mined, allowing scientists access to all samples.
Utilizing assets as a renewable sample resource is a new concept. Instead of using a sample one time due to freeze/thaw constraints, use only a tiny amount. “Science has caught up to the fact that most assays don’t require 4 mL,” Michael explained. The sample can be maximized with a liquid handler for precise aliquoting. Samples like whole blood can be separated and all components stored individually, with annotation to the parent and remaining stock.
Sample-protection and sample-relocation services were among the innovations discussed at the meeting. Sample protection includes sample-storage and sample-preparation services. “We want to help our clients with the renewable sample-resource concept, so we introduced these services,” explained Michael. These include sample processing within 24 hours after collection to maintain sample integrity, sample preparation into precise components (DNA, plasma, etc.), storage at the best temperature for sample type, and tracking of parent/stock sample and child/aliquot relationships via a 21 CFR-compliant database.
Sample-relocation services provide global shipment of samples through the company’s packaging and transportation suppliers and facilities. ReloFleet™ vehicles are repositories on wheels that are temperature controlled and provide door-to-door transportation of sample collections. A real-time sample-tracking database, ISISS (Intelligent Sample Inventory Storage System), provides 24/7 audit trail of sample movement from site to site. “We consolidated over a million samples over four locations and two continents for a pharmaceutical company this year, and it was completed in less than two months,” Michael stated.
Originally developed at Harvard University Medical School, Dale Larson’s automated frozen sample aliquotter drills into frozen specimens and eliminates thawing. “Every time you freeze/thaw a specimen, something happens to it in an unknown, unpredictable manner,” explained Larson, director of biomedical systems at Draper Laboratory.
When establishing a biorepository, he said there are two fundamental approaches in addition to his hybrid methodology for storing and processing samples. The first approach avoids freeze/thaw cycles and aliquots specimens into analysis-size samples for storage. Each sample is analyzed with only one freeze/thaw cycle, but plastic, real estate, and electric costs all increase.
The second approach is to store larger volume sizes, aliquot, and then refreeze after a sample is taken, this may result in a lower quantity of samples. The hybrid approach involves initial freezing in a small number of tubes. The first request thaws the sample and makes aliquots for that particular research activity and the remainder is aliquoted off into multiple tubes.
“What we’ve been able to do with our technology is to get the best of both worlds. You can freeze your sample in a smaller number of tubes initially, then when requested, our system extracts an aliquot without thawing the sample,” Larson explained.
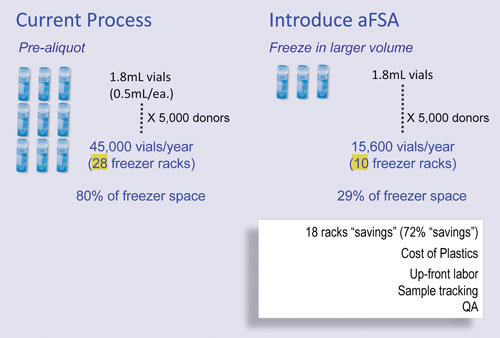
Larson’s hybrid approach consists of a coring probe that drills into the sample and removes it. This enables about six or seven aliquots from a 1.8 mL cryovial, with only one freeze/thaw cycle. Although the robot is not high-speed, it is hands-free. In an economic and labor analysis of the technology, Larson said that out of 24 hours of sample processing at the create-and-deliver aliquot step, 21 hours were returned to the lab, freeing the technician for other tasks.
Research to date has worked with plasma and serum samples in 1.8 mL cryovials, although Larson strongly suspects the system will be able to extract from frozen cells, blood, and soft tissue, but probably not bone. The technology is being further developed by CryoXtract Instruments—a partnership between Harvard and Northeastern Universities.
CryoXtract Instruments was formed to commercialize a robotic system that allows for the automated retrieval of multiple frozen aliquots from a single vial of a frozen biological sample. The firm’s Frozen Sample Aliquotting System is designed to serve the specific needs of biorepositories and biobanks, providing them with a tool to disseminate their serum and plasma samples without exposing them to freeze-thaw cycling.
Turn-Key Biobanking
The huge number of samples being generated for research has created great concern for their accurate and efficient management. Although there are many laboratory information management systems (LIMS) available, they greatly vary in sophistication and robustness.
Thermo Fisher Scientific’s Nautilus LIMS has been on the market for several years, but was recently upgraded with an interface for an outsourcing agent. It was initially integrated with Fisher BioServices, but can be adapted to any partner willing to cooperate and build that end of the webserver, according to Dan O’Donnell, associate director, pharma, Fisher Bioservices.
“What’s different about this system is that it’s a cooperative solution, with both on- and off-site storage samples, along with analytical data. A biorepository system may track data but not analytical data like ours does,” explained Trish Meek, director of product strategy, life sciences, informatics at Thermo Fisher.
The two Thermo divisions partnered to create a sample- and data-management solution by linking the LIMS with internal inventory-management systems. This set-up provides a global view of inventory, centralized web-accessible data, a chain of custody, on-line sample requests, and tracking. In addition, this information can be integrated with laboratory instruments, assay/test requests, and results capture and reporting.
An example of how this may benefit clinical-trial management was presented at the conference. Combined LIMS/IT inventory management provides tools needed to manage the entire process, from sample collection kit configuration to linking of analysis data to the sample. Additional benefits include automatic inventory recording, shipment tracking and visibility, transfer to lab processing, parent/child relationship for aliquots, and on-site/off-site repository inventory view.
“We set up an inventory system for companies that have samples stored with us, which mirrors what their requirements are. For customers that aren’t integrated, we’ll set up a web-service that is a password-protected server where they can find samples and data, request samples to be shipped, and sort data,” O’Donnell added.
A key advantage to this new system is that any new data can be uploaded into the permanent record and become available to everyone within the customer system to view and query. Potential future applications of the integrated system include stem cells and biologic API storage.
Building a Biorepository
There are many factors to consider when building a biorepository. John MacNeela, manager of laboratory operations at Gilead Sciences, provided an overview of key issues. Planning must include the purpose of the biorepository, design of the physical building, required infrastructure, and scope and execution of all operations.
Initial considerations include proximity to air-transportation hubs, commercial real estate costs, availability of critical consumable items (dry ice, liquid nitrogen) via local vendors, and a location close to critical supporting elements required for operation.
Transportation of biospecimens falls under federal and International Air Transport Association regulations. State and local regulations may exist as well depending on the type of biospecimen. Infectious samples that could adversely affect human health due to accidental exposure are considered biosafety level 3 or 4 specimens.
The design of the biorepository must include several factors, including the role of the biorepository, the type of specimens (fluids, tissues, etc.), size, type of HVAC system, back-up generators, and whether to have a liquid nitrogen tank onsite. Storing liquid nitrogen onsite requires more space and the correct plumbing to handle its delivery but reduces costs for delivery. Equipment choices include stand-alone models versus built-in models. Freezer choice depends on the specimens being stored—the colder the required temperature, the higher the cost.
Qualification of equipment and verification of an alarm system are also important. Alarm and thermal-monitoring systems (hard-wired versus wireless) have to be installed and it must be determined whether it will be a single or dual system. Security is a big issue—who has access to core operations and what type of monitoring and surveillance is required. Disaster recovery plans should have additional units for backup/emergency storage, an additional storage facility, and a backup generator.
The decision to close a biorepository should consider a number of points, such as whether it has outlived its original intent, if the original mission has changed, if it is no longer profitable, or if it has sustained extensive damage so the specimen collection has been destroyed, or, if salvageable, needs to be moved to another facility.
Each company must decide whether it is more feasible to outsource specimen storage to a biorepository or make a long-term commitment to onsite storage and operation of a facility, summarized MacNeela.
Sample-Managment Guidelines
There is a well-recognized need for standardized biospecimen guidelines—from sample collection and processing to storage and use in research. The NCI has been working to develop such guidelines through its Best Practices for Biospecimen Resources, Biospecimen Research Network, and caHUB (the Cancer Human Biobank) initiatives.
“We view our role as serving academics, industry, and government. If everyone in R&D is able to move forward more quickly, then patients will benefit,” said Helen Moore, Ph.D., biospecimen research network program manager, office of the director, NCI. A lack of standardized, high-quality biospecimens has been widely recognized as one of the most significant roadblocks to the progress of cancer research.
NCI published its first Best Practices in 2007 to provide guidance for Biobanking, including standardized procedures for collection, processing, storage, and distribution. These guidelines were expanded this year and now include a management and operations section with an expanded informed consent and custodianship section. It is open for public comment on the Federal Register. Dr. Moore said the guidelines will keep changing because the science will continue to change.
“We did a survey of investigators before deciding to build caHUB, and the results were remarkable—the issue of accuracy of research data was resounding. Investigators were clear they really weren’t sure if their results were solid due to variation in specimens.”
The Biospecimen Research Network is funding a study that is evaluating how pre-analytical variables affect the molecular integrity of specimens. There are currently seven investigator-driven studies and one larger study with several investigators. Areas of interest include blood processing, mass spectrometry, as well as protein and gene expression in fixed and frozen tissues.
caHUB will ultimately serve as a centralized, nonprofit public resource for human biospecimens and associated high-quality data acquired via an ethical framework, as well as a source of high-quality Biobanking services. NCI has issued several RFPs—the most recent for collection of cancer tissues, as well as one for informatics and a comprehensive biospecimen resource, which will be awarded to one or more existing facilities. According to Dr. Moore, the initial part of this project is being paid for via stimulus funding. “This is not an area that has been funded in the past, but now there is a lot of job creation by putting these opportunities out to the world.”

caHUB aims to modernize the field of biobanking and contribute to medical advances by providing high-quality human biospecimens and data as well as analysis, scientific tools, and services to the cancer-research and product-development communities.



