October 15, 2009 (Vol. 29, No. 18)
Igor Mezine Ph.D.
Chris Bode, Ph.D.
Sid Bhoopathy Ph.D.
Biomimetic Oxidation Can Facilitate the Generation of Metabolites for Testing Purposes
For at least a dozen years since the publication of a 1997 FDA guidance on in vitro metabolism studies, drug developers have been required to perform in vitro tests of drug interaction potential prior to clinical trials. The 1997 guidance has been updated by a 2006 draft guidance, and, in some cases, it is now advisable to test metabolites as well.
For example, a glucuronide conjugate of gemfibrozil causes a 19-fold increase in the AUC of repaglinide when gemfibrozil is co-administered with itraconazole. Examples such as this highlight the importance of testing for potential drug interactions involving metabolites, in addition to new drugs themselves.
Testing metabolites introduces additional questions and levels of complexity, including:
- Which metabolites to test? (not obvious a priori and impossible to predict)
- Which preclinical species is most appropriate for in vivo testing? In other words, in which species is the metabolite profile most similar to human? (can be determined in vitro)
- Are there any metabolites unique to or disproportionately abundant in humans? (can be determined in vivo)
- How to generate metabolites for testing?
One approach to generating metabolites is biomimetic oxidation, which is currently being applied by Absorption Systems.
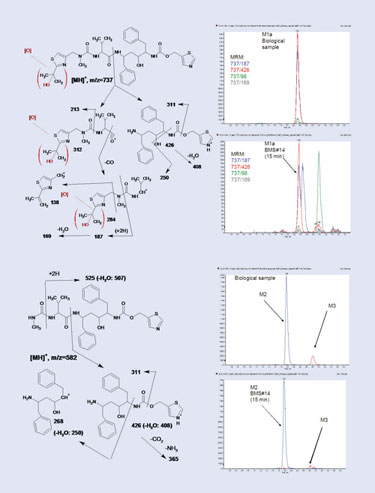
Tentative (bracketed) structure assignments of metabolites M1a (top) and M2 (bottom) based on MS/MS data.
Biomimetic generation of metabolites is based on the use of complexes of transition metals, usually with synthetic porphyrins, to imitate oxidative metabolism catalyzed in biological systems by cytochrome P450 enzymes. For preparative purposes, biomimetic systems offer several advantages over biological matrices, including the fact that organic solvents can be used, which increases the solubility of many drugs; the variability inherent in biological systems is not an issue; scale-up is simple and feasible; and purification of metabolites is greatly simplified by the less-complex matrix.
Ritonavir was used as a model substrate to demonstrate the value of the biomimetic approach. Initially, four different catalysts were tested, along with three organic solvents and two oxygen donors, all at ambient temperature for up to 24 hours. The yield of metabolites was consistently lower when dichloromethane was the solvent compared with acetonitrile or methanol.
Cumene peroxide was a more effective oxygen donor than hydrogen peroxide. Iron-based catalysts, particularly Fe(III)meso-tetra(pentafluorophenyl) porphine chloride, were more efficient in generating mono-oxidative metabolites. The amount of mono-oxidative metabolites peaked at 10 to 15 minutes, then decreased, presumably due to further (not biologically relevant) oxidation.
For the purpose of producing preparative quantities of metabolites, the biomimetic system was scaled up using optimized conditions:
- Solvent: methanol
- Catalyst: Fe(III)meso-tetra(pentafluorophenyl) porphine chloride
- Molar ratio of ritonavir/catalyst/ cumene peroxide: 10/1/50
- Incubation time: 15 minutes
Under these conditions, approximately 50% of the starting amount of ritonavir was recovered at the end of the incubation, along with two biologically relevant metabolites, mono-oxidative M1a (7.8 mg) and N-dealkylated M2 (5.7 mg). A number of other oxidative products were generated biomimetically but were not characterized. The biomimetically generated metabolites M1a and M2 were initially accepted to be identical to the biological metabolites based on their co-elution and indistinguishable MS/MS spectra (Figure). Final structure confirmation was performed by NMR.
Sufficient quantities of metabolites M1a and M2 were produced for in vitro characterization of their potential involvement in drug-drug interactions involving the drug efflux transporter, P-glycoprotein (P-gp). For evaluation of their interaction with P-gp as substrates, bidirectional permeability assays were performed in Transwell® cultures of MDR1-MDCK cell monolayers, a canine renal epithelial cell line transfected with MDR1, the gene coding for human P-gp.
Substrates of efflux transporters such as P-gp have low apical-to-basolateral (A→B) apparent permeability (Papp) values and higher basolateral-to-apical (B→A) Papp values, resulting in an efflux ratio (B→A Papp/A→B Papp) greater than 1. This is due to the fact that P-gp, which is expressed on the apical surface of many epithelial cells, blocks the entry of its substrates across the apical membrane and facilitates the transport of drugs in the opposite direction.
In this manner, ritonavir, M1a, and to a lesser extent M2 (each at a concentration of 5 µM), were identified as substrates of P-gp.
To evaluate their interactions as inhibitors of P-gp, bidirectional permeability assays were performed in Transwell cultures of Caco-2 monolayers, a human intestinal epithelial cell line that naturally expresses P-gp. Digoxin, known to be effluxed by P-gp, was used as the probe substrate.
Inhibitors of P-gp reduce the efflux ratio of digoxin in this assay. Ritonavir completely inhibited P-gp, M1a inhibited by 55%, and M2 was a weak inhibitor. Each compound was tested at a concentration of 20 µM, approximately the plasma Cmax for ritonavir in humans.
It should not be surprising that drug metabolites, like the parent drugs themselves, could potentially be involved in drug interactions as substrates or inhibitors of macromolecules involved in drug disposition. Just as drug metabolites can be pharmacologically active, they can also be pharmacokinetically active. As illustrated here, the drug interaction potential of metabolites cannot necessarily be inferred based on that of the parent molecule. Biomimetic oxidation, a relatively quick and simple means of generating semi-preparative quantities of metabolites, can make the job easier.
Igor Mezine, Ph.D., is senior research investigator, Chris Bode, Ph.D. ([email protected]), is vp of corporate development, and Sid Bhoopathy, Ph.D., is vp of operations at Absorption Systems. Web: www.absorption.com.







