July 1, 2009 (Vol. 29, No. 13)
Susan Aldridge, Ph.D.
Maximizing Viral Safety, Media and Feed Optimization, and Downstream Processing
A number of leading supply and service companies shared their perspectives on biologics production at a recent SAFC Biosciences-sponsored seminar. The topics ranged from cell culture medium design to improving capacities in downstream processing.
Opening the seminar, Martin Wisher, Ph.D., senior scientific director at BioReliance, gave an update on some aspects of European viral and microbial safety regulations. “Guidance documents generally lag behind innovation in products, processes, and scientific discovery,” he observed.
Companies like BioReliance assure viral and microbial safety by testing starting materials, process intermediates, and the ability of downstream processing to clear any contamination. Cell lines tend to be infected with specific viruses that often come from bovine serum (e.g., CHO cells with Cache valley virus and epizootic hemorrhagic disease virus). Dr. Wisher noted that nodavirus infection of insect Tn5 cells is of increasing concern because it can be a mammalian pathogen.
Testing requirements change constantly, with PCR being used to check for new viruses that may not be detectable with standard assays. “A new human or animal virus appears every three months,” said Dr. Wisher. Recent examples include human bocavirus and human polyoma viruses K1 and Wu.
In an attempt to harmonize regulations, EMEA has published guidelines on what viral safety data is needed before going to clinical trials. The guidelines are applicable to all stages of clinical development and will affect companies involved in monoclonal antibodies and certain vaccines. A viral risk assessment of the cell line will now have to be submitted at the same time as the clinical trial application.
“There is a clear move from GLP to GMP as the quality standard for both manufacturing and testing,” Dr. Wisher added. Furthermore, the EU is now saying that master and working viral seed stock must be manufactured to GMP standards, which was not required previously. These proposed changes are currently out for comment.
It finally looks as if the BSE epidemic is burning out. EMEA has updated its guidance on minimizing the risk of transmitting TSEs from human and veterinary products. Where animal-derived materials are used in manufacture, compliance is based upon a risk assessment that aims to minimize, not eliminate, the risk, with traceability of the material being an important element.
Animals must be sourced from BSE-free countries, and derivatives like collagen must come from countries where the BSE risk is known to be controlled. Neither animals nor derivatives can be sourced from a country where the BSE risk is unknown. In addition, blood is now classified as an animal tissue of lower infectivity. Dr. Wisher commented that these changes represent a clarification, rather than a softening, of the rules on TSEs.
“In the future, we will see some exciting new technologies for rapid diagnosis of viral contamination,” Dr. Wisher concluded. Examples include viral detection with rapid PCR, mass spectrometry to detect virus-associated sequences, and multiplex sequencing.
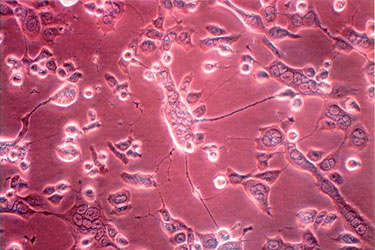
Herpes simplex virus infected Vero cells showing rounded cells and syncytia.
(BioReliance)
Increased Titers
Hikmat Bushnaq-Josting, technical manager at SAFC Biosciences, described how product titers can be increased through media and feed optimization. Titer is related to viable cell density, time in the fermentor, and the productivity of the individual cell. Factors related to the cell itself include the vector used, transfection method, and clone selection, while those related to the process include oxygen, agitation, and pH.
SAFC Biosciences has a CHO cGMP media library reflecting the diversity in clones and clonal nutritional needs. Screening is accomplished either by looking at pure or mixed media or using a more rational design approach based on nutritional depletion, which analyzes spent media. The method chosen depends upon the time available to find an appropriate media for a client project, explained Bushnaq-Josting.
Since every clone is different, this process needs to be done fresh each time. “Each new project for a customer feeds back into the library—we may dispense with some media, but we also add new ones from our genomics and proteomics experiments,” he said.
For instance, in one high-throughput screen of the library, nine CHO cell lines and 30 different media were investigated in 50 mL culture tubes; this showed that for each cell line, use of different media can lead to widely differing performance in cell culture.
Bushnaq-Josting described a case history involving a CHO cell line that produced an IgG for which the customer wanted a 20–30% increase in titer via media development. Four media from the SAFC library gave a higher titer than the customer media, so these were used as the basis for a design-of-experiment approach that involved a pyramid of mixtures. This revealed three mixtures that gave a titer increase of 100–150%—at least at the small scale. The customer found the media selection was scalable to the bioreactor and led to a satisfactory increase in yield to 3,300 mg/L.
One issue that came up in this study was the dilemma of process time. Titer can be improved by increasing culture time in the fermentor. But in a business where every additional day in a large-scale fermentor adds costs, this is not always desirable. There are also questions about the quality of product that spent a long time in the fermentor.
When it comes to feed development, SAFC finds that it is better to look at groups of components and try to assess which ones the culture actually needs most. It is a more time-intensive approach than just adding concentrated media (with all components) but generally gives a better yield of product.
In a second case study, five feed formulations—two complete, three with groups of components—were tested on two clones with two different media using 30 mL bioreactor tubes. The results showed that there is no general rule; the feeds were ranked differently according to performance, depending on the clone and media involved. The best combination gave a yield improved by 67%.
Finally, Bushnaq-Josting described how SAFC, having sequenced the CHO cell line, is now using transcriptome-, proteome-, and metabolome-based tools to optimize cell culture. In one study, SAFC looked at what soy and yeast hydrolysates can add to the performance of a CHO culture. Some CHO genes are expressed in response to both hydrolysates, others uniquely to one or the other. These findings have led to the development of a chemically defined hydrolysate that will be launched soon.
It is hoped that this new product will overcome the problems associated with the fact that although most companies prefer to have a chemically defined (hydrolysate-free) media, it has been observed that many cell lines have a higher titer when being fed with plant-based hydrolysates. With the new CD hydrolysate, the best of both worlds can be combined, explained Bushnaq-Josting.
Downstream Processing
Brendan Fish, Ph.D., director of bioprocess sciences at MedImmune, began his presentation by saying that the difference in complexity between a small molecule and a monoclonal antibody is similar to the difference between a skateboard and a Formula 1 car. He said that although biologic products and processes are complex, fermentation titers are rising and the question has moved to whether downstream processing (DSP) can cope with the new capacity, time, cost, and purity challenges.
An increase in productivity upstream increases the proportion of costs borne by downstream processing. For example, if yields go from 1 g/L to 10 g/L, the proportion of DSP costs rises from 38% to 70%. “Purifying all that protein is a significant burden,” he said.
Other issues impacting downstream processing are the potential purification capacity crunch, the ratio between the number of fermentors and DSP suites, and liquid-handling capacities (large amounts of buffer are required for multiple cycles on large chromatography columns).
These challenges can be met in various ways, Dr. Fish commented, including implementation of platform technologies. In addition, “we are also beginning to understand design space through generation of large process datasets.” Clearly, it is important to keep things simple and avoid nonscalable steps. “It is useful to apply the principles of quality by design, which we are doing increasingly at MedImmune,” Dr. Fish added. Other ways of beating the capacity crunch include the use of disposables and the adoption of high-capacity chromatography matrices.
Chromatography is still the quickest and most cost-effective way to produce large amounts of therapeutic protein. Dealing with large amounts of protein, however, has stimulated the search for improvements to matrix-binding capacity. “The suppliers are aware of the capacity crunch and are trying to help, with several commercial matrices exhibiting capacities in excess of 100 g/L,” Dr. Fish noted.
Multicolumn chromatography is a useful approach that involves collecting fractions and reprocessing them with valve-switching. This process, while new to biotech labs, is common in small molecule development and food technology. MedImmune is also looking at high-throughput process development and at ways of removing aggregates. Other technologies of interest for the future could include precipitation, phase extraction, and monolith columns.
Looking forward, Dr. Fish said that the DSP capacity crunch may not fully materialize, because higher titers might promote a move away from the blockbuster drug model based around large-capacity fermentors. Flexibility and appropriateness of facility scale combined with DSP technological advances are likely to drive the biotech processes of the future.
Membrane Technology
Finally, John Moys, head of technical support for North Europe at Sartorius Stedim Biotech, discussed some of the advantages of membrane technology, where major improvements are predicted. He specifically focused on the Sartobind membrane adsorber technologies for which many different chemistries are available now.
“Membranes are better than conventional bead technologies for polishing because you get a higher flow rate with the open structure,” Moys said. A comparison study carried out at Amgen has shown that membranes are quicker (2–2.5 hours versus 8–9 hours) than gels and take less buffer in polishing of a monoclonal antibody. “Scale-up of membranes is still challenging, but is being addressed,” he added.
The Sartorius Stedim Hydrosart® ultrafiltration cassette range is a cellulosic membrane with low protein adsorption that can be cleaned with sodium hydroxide at up to 1 molar concentrations at elevated temperatures. In virus removal, an orthogonal approach is now needed before Phase I—the technologies being used are UVC inactivation, chromatography, and nanofiltration.
For UVC, inactivation must be done without damaging the product—the UVivatec® achieves this through a helical reactor design that allows uniform irradiation through radial mixing based on Dean vortices, Moys said. This technology was developed by Bayer Technology Services and is exclusively distributed by Sartorius Stedim Biotech.
With nanofiltration, Moys said that there can be a fine line between virus and product, with biomolecules having the potential to block a filter. Virosart® is a disposable nanofilter that can give a log reduction value of virus of greater than four, he explained. For final filtration, the latest advances involve polyethersulphone membranes that allow higher surface area and therefore a higher flow rate and throughput performance.

Sartorius Stedim Biotech’s Hydrosart membrane is a stable polymer that features a broad pH and temperature range.
Susan Aldridge, Ph.D. ([email protected]), is a freelance science and medical writer.



