October 1, 2011 (Vol. 31, No. 17)
Innovative World of Biopharmaceutical Manufacturing
Our main focus is to create brighter lives for people today and for generations to come. With more than 25 years of experience in contract manufacturing DSM Biologics has the essential expertise and drive to help our customers in their mission to save human lives. DSM offers dedicated services and by using a variety of technologies we manufacture our customer’s new generation drugs for treatment of incurable diseases, e.g., various types of cancer and autoimmune diseases.
Innovation is the main driving force in exceeding our customers’ expectations. Challenging conventional process technologies with innovation and forward-looking solutions leads us to the next generation of manufacturing processes—leaner and more efficient.
Our state-of-the-art facility, located in Groningen, The Netherlands, is dedicated to the development, scale-up, and cGMP manufacturing of recombinant proteins and monoclonal antibodies. The Groningen facility has consistently been successful when inspected by European and U.S. regulatory authorities. In order to meet the global needs of our customers DSM Biologics is currently expanding its manufacturing capability into new geographic area. Our new facility for cGMP manufacture of biopharmaceuticals will be open in 2013 in Brisbane, Australia.

Markets
Experienced with a global customer base DSM Biologics provides manufacturing technologies and services to the worldwide biopharmaceutical market. Being in the industry for more than a quarter of a century the company has adapted its services to the needs of our customers and to evolving challenges in the biopharmaceutical market. Today we partner with small, medium, and large pharmaceutical and biotechnology companies, and support them from the earliest stage of development, through clinical trials and to full commercial supply.
Capabilities
DSM Biologics offers state-of-the-art facilities and contract manufacturing expertise to our customers, and delivers the right quantity and quality of biopharmaceutical products within the agreed customer timelines. Our service portfolio includes manufacture of therapeutic recombinant proteins, monoclonal antibodies, fusion proteins, and antibody fragments.
Our Groningen plant is designed to address all of our customer needs. The plant comprises process development and cGMP facilities of ~20,000 ft2 (~1,860m2) each. The cGMP area contains suites with 50 L, 250 L and 1,000 L single-use and stainless steel bioreactors, as well as several DSP suites equipped with filtration devices (dead-end, cross-flow filtration) and all of the necessary types of chromatography operation (resins or membrane). DSM Biologics provides a full range of analytical development capabilities as well as regulatory support to all customers.
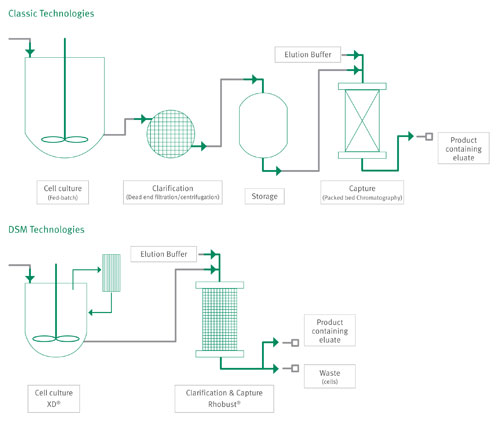
DSM Biologics’ unique position in being able to utilize innovative and superior process technologies, like the proprietary XD® and Rhobust® technologies (above), makes the company a sought-after technology provider.
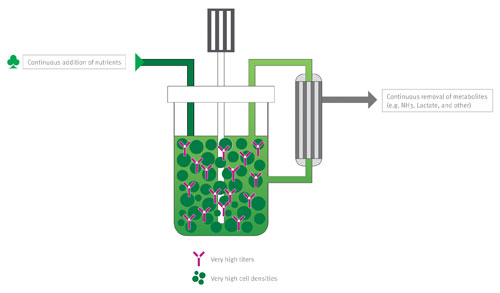
The XD technology is a highly intensified cell culture process, that provides cells with a constant environment for optimal cell growth. The XD Technology works in a continuous media feeding mode, with a filtration unit to retain both the cells and the recombinant protein in the bioreactor (right). The process is robust and scalable, while still maintaining consistent product quality. This revolutionary technology for mammalian cell culture results in 5- to 15-fold increases in titers compared to the original process.
Rhobust technology is a second generation expanded bed adsorption (EBA) technology that provides an elegant solution for direct capture in the cell separation unit operation, by combining clarification and product capture into one single step. Centrifugation, depth-filtration, and packed bed chromatography can be replaced by one unit operation, resulting in less preparation and process time, lower cost of goods, and reduced investment costs. In addition, product recovery and purity are equally good or better to classical process approaches.
We invite you to hear more about the exciting innovations and services available at DSM Biologics by emailing [email protected] or calling 973-257-8220 for a personal follow-up from our team.
DSM Biologics
45 Waterview Boulevard
Parsippany, NJ 07054-1298
Phone 973.257.8220
Email [email protected]
Website www.dsmbiologics.com
Date Founded 1986
Number of Employees 160






